
Concept explainers
The chapter sections to review are shown in parentheses at the end of each problem.
Balance each of the following by adding coefficients, and identify the type of reaction for each: (8.1, 8.2, 8.3)
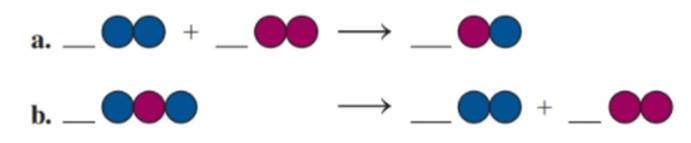
(a)
Interpretation:
The given reaction should be balanced by adding appropriate coefficients; in addition the type of the reaction needs to be identified.
Concept Introduction:
- Chemical reactions can broadly be classified into 5 types based on how the reactants interact to form products, these include: combination reaction, decomposition reaction, single replacement, double replacement and combustion reaction.
- A chemical reaction is said to be balanced if there are equal number of atoms of one kind in the reactants and products
Answer to Problem 35UTC
If blue spheres = A, red spheres = B, then the balanced equation is:
This is a combination reaction
Explanation of Solution
In the given reaction, two molecules combine to form a single product. This is representative of a combination reaction.
Let the blue spheres be represented by the element ‘A’ and the red by element ‘B’. The given unbalanced reaction is therefore,
The equation is not balanced since the number of A and B atoms in the reactants are 2 while there are only 1 each on the product side. In order to balance the equation, multiply BA by 2 to get:
(b)
Interpretation:
The given reaction should be balanced by adding appropriate coefficients; in addition the type of the reaction needs to be identified.
Concept Introduction:
- Chemical reactions can broadly be classified into 5 types based on how the reactants interact to form products, these include: combination reaction, decomposition reaction, single replacement, double replacement and combustion reaction.
- A chemical reaction is said to be balanced if there are equal number of atoms of one kind in the reactants and products
Answer to Problem 35UTC
If blue spheres = A, red spheres = B, then the balanced equation is:
This is a decomposition reaction
Explanation of Solution
In the given reaction, a single reactant splits to form two simpler compounds. This is representative of a decomposition reaction.
Let the blue spheres be represented by the element ‘A’ and the red by element ‘B’. The given unbalanced reaction is therefore,
The equation is not balanced since the number of A and B atoms in the reactants are 2 and 1 respectively while in the product side they are 2 each. In order to balance the equation, multiply ABA by 2 and AA by 2 to get:
Want to see more full solutions like this?
Chapter 8 Solutions
Basic Chemistry
- How much heat (in kilojoules) is evolved or absorbed in the reaction of 1.65 gg of NaNa with H2OH2O?2Na(s)+2H2O(l)→2NaOH(aq)+H2(g) ΔH∘2Na(�)+2H2O(�)→2NaOH(��)+H2(�) Δ�∘ = -368.4kJkJ.arrow_forwardDuring the combustion of 5.00 gg of octane, C8H18C8H18, 239.5 kcalkcal (1002 kJkJ) is released. what is the balanced equation for the combustion reaction? How much energy (in kJkJ) is released by the combustion of 1.21 molmol of C8H18C8H18 ? How many moles of octane must be burned to release 442.4 kcalkcal ? How many kilocalories are released by the combustion of 18.3 gg of C8H18C8H18 ?arrow_forwardA 27 g aluminum foil pan is used to roast vegetables. The pan is put into a cold oven at 22 oC . How much energy in cal is absorbed by the pan after cooking at 232 oC (450. oF) for 25 minutes?arrow_forward
- Which of the following correctly describes the values of % AE and the E-factor for the reaction below? (% AE = percent atom economy; E-factor = environmental factor) pick one option 4 NH3 + 5 O2 → 4 NO + 6 H2O % AE < 100; E-factor = 0 % AE = 100; E-factor > 0 % AE = 100; E-factor = 0 % AE = 0; E-factor > 0 % AE = 0; E-factor = 0arrow_forwardA student reacted 1.01g salicylic acid with 2.06mL acetic anhydride and isolated 1.40g aspirin. A) Calculate moles of acetic anhydride used. B) Using the moles of limiting reactant present, calculate the theoretical yield of aspirin. C) Calculate the percent yield of the aspirin synthesis.arrow_forwardWhat is Ka, Kb, relationship between these and Kw.arrow_forward
- a 20 mg sample CxHy is burned in oxygen to produce 60 mg of carbon dioxide and 32 mg of water. calculate the value of x and y.arrow_forwardThe table below shows Hvalues for 3 reactions. Which reaction has Hrxn equal to Hf? Explain. How much energy would be released by the combustion of 100.0 grams C2H2(g)? Calculate the ΔHrxn for the reaction C2H2(g) → 2C(s) + H2(g).arrow_forwardAt 714 oC, Keq = 1.78 for the reaction: SO2(g) + 1/2 O2(g) SO3(g) (a) What is the value of Keq for the reaction SO3(g) SO2(g) + 1/2 O2(g)?Keq = .(b) What is the value of Keq for the reaction 2 SO2(g) + O2(g) 2 SO3(g)?Keq = .(c) What is the value of Keq for the reaction 2 SO3(g) 2 SO2(g) + O2(g)?Keq = .arrow_forward
- Draw a potential energy diagram for a system in which the forward reaction has Eact = +42 kcal/mol and the reverse reaction has Eact = +28 kcal/mol. a. Is the forward process endothermic or exothermic? ["", ""] b. What is the value for ΔH for this reaction? Please insert an image of your workarrow_forwardWhich of the following correctly describes the values of % AE and the E-factor for the reaction below? (% AE = percent atom economy; E-factor = environmental factor) 3 CO + 7 H2 ⟶ C3H8 + 3 H2Oarrow_forward2. The following questions are related to the production of hydrogen chloride from hydrogen and chlorine, as shown here:H2 (g) + Cl2 (g) ⇄ 2 HCl (g) a) What is the value of H˚ at 25 ˚C? b) What is the value of S˚ at 25 ˚C? c) What is the value of G˚ at 25 ˚C?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
