What is Metallurgy?
Metallurgy could be defined as a technique by which metals can be extracted in their pure nature. Extractive metallurgy directs with the route of eradicating the valuable metals from ores and purifying the extracted metals to pure nature. In hydrometallurgy, the metal extraction’s from their ores is done using an aqueous solution. In the pyrometallurgical process, high temperatures are employed to initiate the reactions between gases, molten metals, and solids. In electrometallurgy, the technique is carried in an electrolytic cell.
Refining of a crude metal
In metallurgy, a vital part is involved by refining. An impure metal is gotten from its ore. The extracted impure metal is crude metal. The technique of converting an impure metal to a pure form is the refining of crude metal. When an impurity is isolated from crude metal through the refining process, metals with high purity could be attained. During the refining method, the end-product obtained is analogous to the real substance whereas it could be in pure form. In simple words, the crude metal’s purification is refining.
Methods for purifying the crude metal
Several techniques are prone to eliminate the impurity from crude metal. The technique of metal refining could get differs on basis of the metal’s nature and use, & also based on the physical characteristics of impurities. Some of the refining processes are:
- Distillation.
- Poling.
- Liquation.
- Zone refining.
- Electrolytic refining.
- Chromatographic method.
- Vapor phase refining.
- Cupellation.
Distillation
The technique of isolating the constituents on basis of the dissimilarity in the boiling point is distillation. In distillation, the impure metal is vaporized to acquire the metal’s pure nature as distillate. Metals like mercury (Hg) & zinc (Zn) possess a lesser boiling point. When metals like zinc, mercury are heated above their boiling point, they get vaporized completely. The impurities (solid) are leftover. The vapor, which contains metal in pure form is condensed and is gathered as a pure metal.
Poling
The procedure of purifying those metals that comprise oxidized impurities is poling. Metals like copper or tin in the impure form of copper oxide or tin oxide are purified. In ancient times, branches of trees that are green were needed to blend the molten metal as the hydrocarbon organic substances found in branches are helpful for the reduction of oxide impurities.
During poling, impure copper is preserved in an anode incinerator for the refining process. When air is blustered, sulfur & iron are liberated as sulfur oxide & iron oxide respectively. Oxides of iron are liberated from copper & through the off-gas system of the incinerator; & sulfur oxide is emitted out. Once after the initial oxidation stage, poling is the next stage. The oxide from cuprous oxide is eliminated through natural gas or diesel (reducing agent) and copper in a pure state is acquired.
Liquation
The technique of splitting the substances of alloy or crude metal or ore through partial melting is liquation. The technique of liquation is comparable to distillation. In the liquation method, the metal’s melting point (MP) is observed. Liquation is utilized to purify metals with lower melting points. The metal’s melting point would be less than the impurity’s melting point. At the higher temperature, on supplying heat to the metal, the metal starts to melt whereas the impurity is solid. Henceforth, it is easier to segregate the impurity from the pure metal. Generally, the liquation is carried in a sloped container, so the liquid metal flows down & the impurity is a residue in the container.
Zone refining
Silicon, germanium, gallium, boron, & indium are the metals that could be refined by zone refining. When impurities are found, the material’s melting point would be less. Impure metal is made as a bar. Around a bar, the circular heater is attached & is taken from one terminal to another. The bar starts to melt because of the heater, crystals of pure metal are acquired & the impurities are lifted to the nearby molten region. The impurity is wiped from one to the other region, & metals with high purities are obtained.

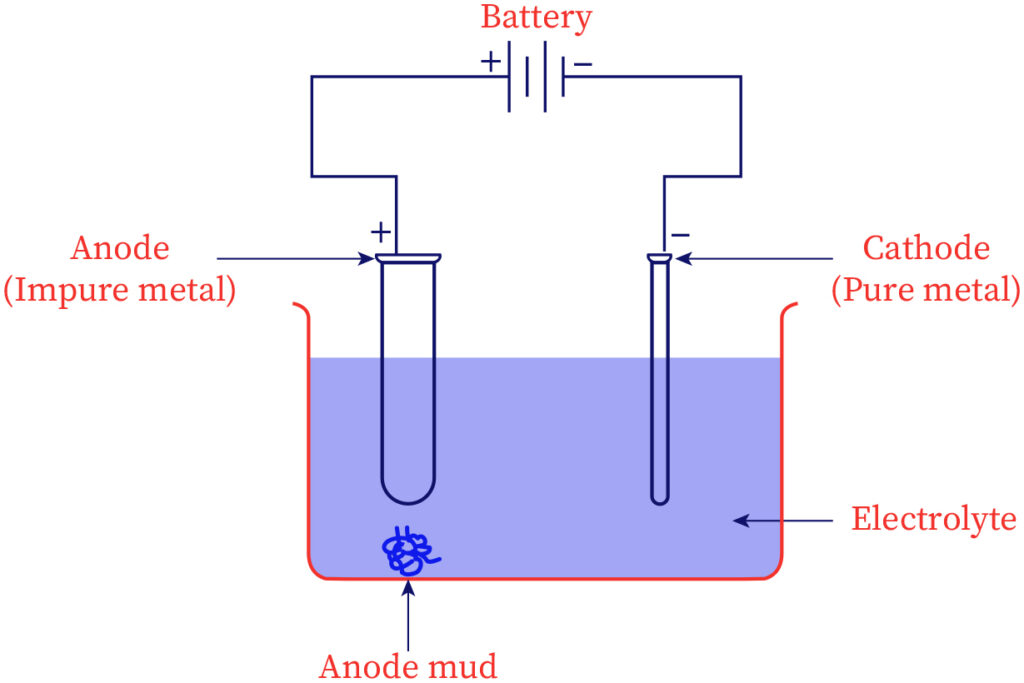
Electrolytic refining
The most diversely used process to purify several metals is electrolytic refining. Electrical energy is employed for breaking down the chemical bonds between impurities and impure metal. The anode contains impure metal & in the cathode, the sheet of metal in the pure state is placed. An electrolytic solution or electrolyte is a solution containing salts of the same metal. On passing electricity over the solution, the ions of metal from the electrolytic solution get set down in the cathode as pure metal whereas an equal mass of metal dissolves from the anode to the electrolyte as ions of metal. Copper, aluminum, tin, zinc, etc could be refined through this process.
Chromatographic methods
The chromatographic methods are on basis of individual adsorption of several constituents of a mixture on an adsorbent. The mixture is placed on a gaseous medium or liquid that moves through the adsorbent.
The adsorption of different constituents is at several stages in the column. Using the required eluants (solvents), the constituents, which are adsorbed, are given out. Based on the physical nature of the stationary phase and moving phase, the chromatographic routes are given several names like gas chromatography, thin-layer chromatography, paper chromatography, and column chromatography.
Vapor phase refining
In the vapor phase refining process, metals react with a suitable reagent to yield a volatile substance. Decomposition of the volatile substance helps to recover the metal.
Nickel could be refined using this method. Volatile nickel carbonyl (Ni(CO4)) is formed when nickel undergoes heating in carbon monoxide. When nickel carbonyl goes through thermal decomposition, pure nickel is obtained. Titanium, zirconium, etc could be also be refined by vapor phase refining.
Cupellation
The technique employed for the purifying silver comprising of lead impurity is cupellation. This procedure is on basis that the oxidation or chemical reactions of precious metals do not happen. At higher temperatures on heating precious metals, they stay apart whereas slag is formed when other components chemically react.
Difference between refining and extraction of metals
The technique through which metals are extracted from their ores is the extraction of metals whereas refining is the technique by which pure metals are gotten after the extraction process. The purity of the metal could be increased through refining.
Context and Applications
This topic is vital for bachelors in chemistry, and for bachelors in metallurgical engineering.
Practice Problems
Question 1: The vapor phase refining of nickel is _____.
- Mond process
- Van Arkel method
- Haber’s process
- Electrolysis
Answer: Option 1 is correct.
Explanation: Nickel is purified by the Mond process. A volatile NiCO4 is formed when nickel is heated with carbon monoxide. Pure nickel is recovered, when NiCO4 gets decomposed.
Question 2: Name the electrolyte, which is utilized in refining copper.
- Copper nitrate
- Copper sulfate
- Carbon
- Copper sulfide
Answer: Option 2 is correct.
Explanation: The electrolyte needed for purifying copper must be a soluble salt solution of copper such that ions of copper could be deposited as pure copper. Copper sulfate is taken as the electrolytic solution.
Question 3: Name the process that does not involve purifying the metal.
- Froth flotation
- Poling
- Distillation
- Zone refining
Answer: Option 1 is correct.
Explanation: Zone refining and distillation are used to purify metals whereas froth flotation is done to eliminate gangue from ores of sulfides.
Question 4: Among the given options, aluminum is refined by____.
- Bosch process
- Hoopes process
- Mond process
- Baeyer’s process
Answer: Option 2 is correct.
Explanation: A metallurgical route for obtaining high purity aluminum metal is the Hoopes process.
Question 5: Among the given options, pure tin cannot be gotten from ____.
- Poling
- Liquation
- Zone refining
- None of the above
Answer: Option 3 is correct.
Explanation: As the melting point of tin is low, zone refining is not used in refining tin.
Want more help with your chemistry homework?
*Response times may vary by subject and question complexity. Median response time is 34 minutes for paid subscribers and may be longer for promotional offers.
Refining of Crude Metal Homework Questions from Fellow Students
Browse our recently answered Refining of Crude Metal homework questions.