What is the Freezing Point of Water?
In general, the freezing point of water is 0° Celsius, or 32° Fahrenheit. This is the temperature at which water will ordinarily change from its liquid state to its solid state (ice). However, there are certain conditions that can affect the freezing point of water. For example, a liquid may be supercooled or contain impurities so that it does not freeze at the ordinary freezing point.
What is Happening Chemically When Water Freezes?
Similar to the boiling point, where water changes from the liquid state to the gaseous state, the phase transition of a material from liquid to solid is referred to as freezing. This is a phase transition phenomenon, which means the material changes from one state of matter to another.
Water molecules are closely bound, and the intermolecular forces of attraction are weaker in liquids than in solids. This is because the thermal energy added to the molecules during melting (the process of converting from a solid to a liquid state) is greater than the potential energy of the molecules binding them together in a solid's crystal lattice. This energy is also representative of the solid's lattice energy. As the temperature of a material is reduced by withdrawing heat or lowering the pressure, the molecules lose kinetic energy and move closer together. They gradually accumulate potential energy and stabilize. They are transformed to solid at this stage. This is a stable form of water.
How Does Something Freeze?
Freezing Point
The freezing point of a substance is the temperature at which it transforms from liquid to solid at ambient pressure. At the freezing point, the two phases, liquid and solid, are in equilibrium, which means that both solid and liquid states occur at the same time. A substance's freezing point is affected by air pressure.

How Freezing Happens
When the temperature drops below a certain point, the liquid state transforms into a solid state. That particular temperature is known as the freezing point for that particular liquid. The freezing point varies from one liquid to another.
In a substance's liquid state, molecules are loosely packed and move freely due to lower intermolecular force of attraction. When the temperature decreases and cooling starts, the liquid starts losing thermal energy. This results in a decrease in molecular activity, and the molecules start to come close to each other. When freezing starts, the molecules settle in place, and attractive forces keep them together. Crystal formation starts, which we see as ice on the water surface.
The freezing temperature of water can be affected by dissolving solvents in the water, and hence it finds application in various industries.
Note:
During the freezing process, there is no change in temperature, and solidification of liquid starts. There is an energy release during this process.
Factors Affecting Freezing Point

Types of molecules: The freezing point of a substance is determined by the kinds of molecules that comprise it. The freezing point would be relatively low if the intermolecular forces between molecules are relatively strong. For example, methyl ether has a freezing point of -138.5 ℃, while ethyl alcoholhas a freezing point of -117.3 ℃.
Surrounding effects: Environmental factors like wind can have an affect on the freezing point. This is because it can affect the heat transfer rate from the liquid. With an increase in wind speed, the heat transfer coefficient increases, which result in great heat losses from the liquid, hence faster cooling.
Freezing Point Depression
Freezing point depression is a decrease in the temperature at which a material freezes caused by the addition of a smaller quantity of another, non-volatile substance. Salt in water (used in ice cream makers and to de-ice roads), alcohol in water, ethylene or propylene glycol in water (used in automotive antifreeze), or the combination of two solids, such as impurities, into a finely ground drug are all examples.
Seawater (a mixture of salt and other additives in water) remains liquid at temperatures below 0 °C (32 °F), which is the freezing point of pure water. Hence salt water can be used for industrial purposes.
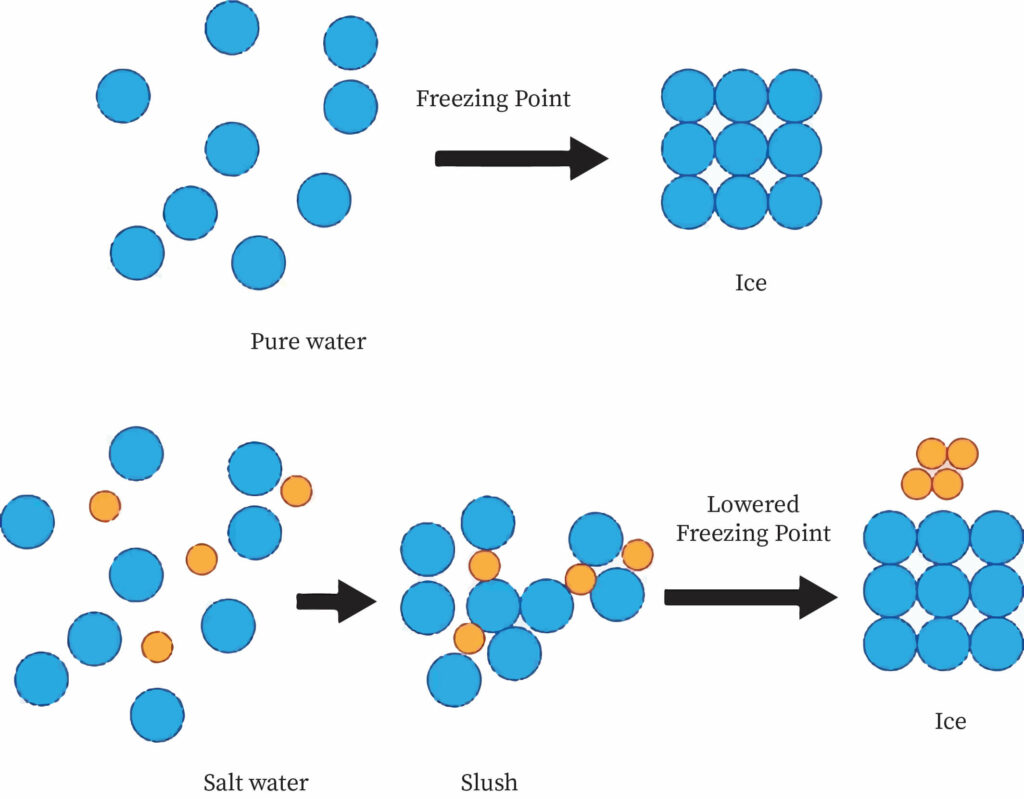
Reasons for Freezing Point Depression
Similar to boiling point elevation, when a non-volatile solute is mixed with a volatile liquid solvent, the solution vapor pressure is smaller than the pure solvent. As a result, the solid will come into equilibrium with the solution at a lower temperature than the pure solvent. Since a vapor's chemical potential is logarithmically proportional to heat, this explanation in terms of vapor pressure is similar to the logic based on chemical potential. Many of the colligative properties derive from a decrease in the solvent's chemical potential in the presence of a solute.
Common Misunderstanding
A substance's freezing point is the same as its melting point, which can sometimes cause confusion. While a freezing point is the point at which a liquid turns to a solid, a melting point is observed when solid changes to a liquid state. That is, a substance will change from liquid to solid state or from solid to liquid state at the same temperature.
Context and Applications
The freezing point of water and freezing point depression are important concepts that are used often in day-to-day life. Here are just a few real-world examples:
- Automobiles: A car's radiator fluid is a combination of water and ethylene glycol. In the winter, freezing point depression prevents radiators from freezing.
- Civil: Road salting uses this effect to lower the freezing point of the ice on which it is applied. Lowering the freezing point causes street ice to melt at lower temperatures, avoiding harmful, slick ice build-up.
- Biological: Any species that survives in deep cold utilizes freezing point depression. These species have evolved mechanisms for producing high concentrations of various compounds, such as sorbitol and glycerol. This increased solute content lowers the freezing point of the water inside them, stopping the animal from freezing solid even as the water surrounding it freezes or the air around it becomes very cold. Some arctic fish, such as the rainbow smelt, develop antifreeze compounds to survive through the winter.
- Chemistry: When tested using differential scanning calorimetry, freezing-point depression can also be used as a purity measurement technique. While the findings are in mol percent, the system has a spot where most methods of study lag.
- Food industry: This phenomenon is applicable in the preparation of a freezing mixture for the production of ice cream. NaCl or another salt is used to lower the melting point of ice for this reason. Freezing point depression tests are often used in the dairy industry to ensure that no additional water has been applied to the milk. Unadulterated milk has a freezing point of more than 0.509 °C.
Want more help with your physics homework?
*Response times may vary by subject and question complexity. Median response time is 34 minutes for paid subscribers and may be longer for promotional offers.
Freezing Point of Water Homework Questions from Fellow Students
Browse our recently answered Freezing Point of Water homework questions.