1) Find the pH of 0.1 M of the differnet forms histidine species. (See image for equation and pKa values) 2) What is the principal specis at pH 1, pH 5, pH 8, and pH 11?
Q: Select one: O a. will require an input of energy O b. O c. will absorb energy O d. is not a…
A: The Gibbs–Helmholtz equation is a thermodynamic equation which is used calculating changes in the…
Q: Glycogenin is a homodimer of 37 kDa subunits. (Homodimer means identical subunits of type a that…
A: Glycogenin: It is a transferase that produces glycogen, which is a crucial type of glucose storage…
Q: 3. Compare and contrast how thiolase and chymotrypsin creates and stabilizes its intermediates.
A: Enzymes are highly specialized proteins that have extraordinary catalytic power, greater than that…
Q: What is the complete base composition (in %) of a double-stranded eukaryotic DNA that contains 16%…
A: Introduction DNA acts as a genetic material in our body. It is a self replicating molecule. DNA…
Q: how GABA balances glutamate to prevent seizures and what is one GABA drug that is used to do that.…
A: Glutamate is a precursor of GABA. The enzyme glutamate decarboxylase catalyzes the decarboxylation…
Q: Experiment: Lipids Qualitative Tests Test Performed: Translucent Test Procedure 1. Fold your…
A: Lipids are a chemically diverse group of biomolecules with two common characteristics: low…
Q: In beta-oxidation, which cofactor is required the for second oxidation reaction (conversion of…
A: Fatty acid β-oxidation is the metabolic process by which fatty acids are broken down to produce…
Q: a) What is the overall function of pyruvate carboxylase? b) Describe the role of each of the four…
A: (a) A metabolic enzyme called pyruvate carboxylase takes part in the first stage of gluconeogenesis.…
Q: 5. Describe the role of His in the catalytic mechanism shown.
A: Enzymes are highly specialized proteins that have extraordinary catalytic power, greater than that…
Q: Why doesn't the net reaction for the citric acid cycle have intermediates (citrate, isocitrate,…
A: The acetyl CoA molecules produced through carbohydrate, lipid, and protein metabolism undergo…
Q: Draw the fractional binding curve with protein that bind to a molecule of ligand L.
A: A ligand is a molecule that binds to a receptor. The specificity of the ligand varies. One ligand…
Q: The ability of hemoglobin to deliver oxygen is enhanced because: A) The partial pressure of oxygen…
A: Hemoglobin is an oxygen-transporting protein that transports oxygen to most tissues. Hemoglobin is…
Q: Briefly explain chymotrypsin with reference to the following: a) Catalytic triad (not the mechanism…
A: DISCLAIMER FOR MULTIPART Since you have posted a question with multiple sub-parts, we will solve…
Q: Give an example of how Transamination takes place by a chemical reaction.
A: In transamination reaction, amino group is transferred from a donor substrate to an acceptor alpha…
Q: BIOC 384 Structure and Function of Simple Sugars Q9.1: NutraSweet (aspartame) is not a carbohydrate…
A: Carbohydrates are organic molecules that are composed of carbon, hydrogen, and oxygen. They are…
Q: Chemical mutagens often cause oxidation or deamination of DNA bases. This can lead to cancer by…
A: DNA damage is often caused by external sources like radiation exposure, exogenous chemical reactions…
Q: 1. Minor changes in primary structure can have pronounced effects on biological function. Tetrameric…
A: Hemoglobin is a tertameric protein made up of 4 subunits namely α2β2. The αβ form a dimer and 2 such…
Q: Identify the reasons why the DNA molecule you selected would lead to DNA synthesis. It is…
A: An important characteristic of DNA replication is that it is semiconservative. Two strands of DNA…
Q: The structure of a metalloenzyme active site is down below(black picture). Describe, from a chemical…
A: Methane monooxygenase (MMO) is present in methanotrophic bacteria and MMO is present in an integral…
Q: 5. Anaerobic glycolysis: definition, localisation, biological significance.
A: All living organisms go through cellular respiration, which begins with glycolysis. In the cytoplasm…
Q: The steady-state kinetics of an enzyme are studied in the absence and presence of an inhibitor B…
A: Enzymes are protein molecules that increase the rate of reaction by decreasing the activation…
Q: 3. Pyrozole has been proposed as a possible nontoxic inhibitor of LADH-catalyzed ethanol oxidation.…
A: Since you have posted a question with multiple sub parts, we will provide the solution only to the…
Q: Based on what is known about the mechanism of Chymotrypsin, which molecules would be inhibitors of…
A: Enzymes are highly specialized proteins that have extraordinary catalytic power, greater than that…
Q: Iodoacetate reacts irreversibly with the free -SH groups of cysteine residues in proteins. List…
A: Calvin-Benson-Bassham cycle is commonly known as the Calvin cycle or Dark reactions of…
Q: Summarize the background information about the enzyme b-galactosidase ,protein purification in…
A: Enzymes are biological catalysts that increase the rate of a biochemical reaction. Enzymes do not…
Q: Question 14 The first three amino acids in a protein are lysine, glutamine, and serine. T describes…
A: The four classes of biological macromolecules are proteins, nucleic acids, carbohydrates and lipids.…
Q: The second step during elongation of fatty acid chain is catalyzed by B-ketoacyl reducatse. In which…
A: Simple fats or triglycerides or triacylglycerides are categorized as storage lipids. Triglycerides…
Q: Calculate the appropriate volume (in µL) of 9X loading buffer that should be added to 46.0 µL of a…
A: Agarose gel electrophoresis is a laboratory technique that is used for the separation of nucleic…
Q: reaction= Acetaldehyde +NADH +H+ ethanol +NAD+ Describe two procedural changes that would have…
A: The end product of glycolysis is pyruvate. Pyruvate has two possible fates: in presence of oxygen,…
Q: Vasopressin is a hormone that plays an important role in social behavior, sexual motivation, and…
A: Recall that: Amino acid sequences are written with N-terminal amino acid on the left and…
Q: The difference between purine and pyrimidine systhesis is: One builds the base on top of the sugar…
A: Among the nucleotides, adenine and guanine are purines. Cytosine and thymine are pyrimidines.…
Q: Acetyl COA (ACC) is a critically important molecule in metabolic pathways. This molecule can be used…
A: ACC is the rate limiting enzyme of fatty acid synthesis pathway. ACC catalyzes the conversion of…
Q: 3. Attach the structures below to draw a sphingolipid. CH3-(CH2) 12-CH=CH-CH-OH sphingosine CHINH,…
A: Lipids are a chemically diverse group of biomolecules that have two things in common: low…
Q: The structures are tautomers of nucleotide bases. Identify each base. OH Base: OH ☆ Base: Base: "OH…
A: Nucleic acids are composed of nucleotides (sugar, nitrogenous base & phosphate group). Nucleic…
Q: Which of the following is a ketone body? O Pyruvate Acetoacetic acid O Lactic acid O Glycogen
A: Ketone bodies contain ketone groups and are produced in the liver from fatty acids. The process of…
Q: What else? What are cofactors? Carboxypeptidase requires a Zn²+ cofactor for the hydrolysis of the…
A: Since in the question it is mentioned to answer any three questions, question 2, 5 & 6 will be…
Q: 1 is there a positive or negative entropy change in the first step of histidine synthesis? 2 how…
A: "Since you have asked multiple questions, we will solve the first three questions for you. If you…
Q: Which of the following describes the interaction between the amino acid last eluted and the anion…
A: In Ion exchange chromatography the molecules are separated according to their charge. The matrix…
Q: Which of the designations are accurate for the fatty acid?
A: Simple fats or triglycerides are fatty acid esters of glycerol, where three fatty acid molecules are…
Q: After 7 rounds of b-oxidation completely converts fluorooleate into acetyl-CoA, draw the molecule…
A: Fatty acids are carboxylic acids with a hydrocarbon chain ranging from 4 carbon to 36 carbons.…
Q: Which is CORRECT for the flow of electrons from NADH? NADH Complex I→ ubiquinone → Complex III →…
A: The electrons from NADH is transferred via a series of complexes to synthesize ATP. This is known as…
Q: . What is the nucleotide sequence of the complementary strand of the DNA molecule:…
A: DNA is the genetic material in most organisms. During transcription RNA Polymerase synthesizes the…
Q: The iron (Fe) ion in the heme group of hemoglobin is bound to a: OA) Cystine B) Histidine C) Proline…
A: Our red blood cells (RBCs) are composed of hemoglobin that helps to transport oxygen throughout the…
Q: I. II. III. IV. O HẲN-CH-CANH-CH,C-NH-CH-CƠ V. O CH3 alanyl 2. Types of protein structures: What is…
A: "Since you have posted a question with multiple sub-parts, we will solve the first three sub-parts…
Q: Mechanistic probes often require the use of an electrophilic functional group to interact with an…
A: A nucleophile is a chemical species that is negatively charged or has a high electron density or a…
Q: 4. Consider the reaction: H3C OH CH C H3C C H₂ a. What kind of reaction is being performed here? b.…
A: Fatty acids are carboxylic acid with an aliphatic chain that can be saturated or unsaturated. The…
Q: 3. Question from Lehninger...describe the common structural features and the differences for each of…
A: Carbohydrates or carbs are maconutrient consisting of Carbon, hydrogen and oxygen atoms. They are…
Q: these lipids are soluble with dichloromethane? : olive, oleic acid, stearic acid, Lecithin, Vitamin…
A: Lipids are substances that are insoluble in water and soluble in organic solvents such as alcohol…
Q: Phosphoenolpyruvate (PEP) is converted to pyruvate by the enzyme pyruvate kinase. The standard free…
A: The standard free energy change (∆Gº') is the amount of energy released in of biochemical reaction…
Q: A/client plans to go foreah10-mile rum, but they become fatigued aftein mites, so they stop: What is…
A: Cellular respiration is a collection of three metabolic pathways that generate ATP the energy…
1) Find the pH of 0.1 M of the differnet forms histidine species. (See image for equation and pKa values)
2) What is the principal specis at pH 1, pH 5, pH 8, and pH 11?
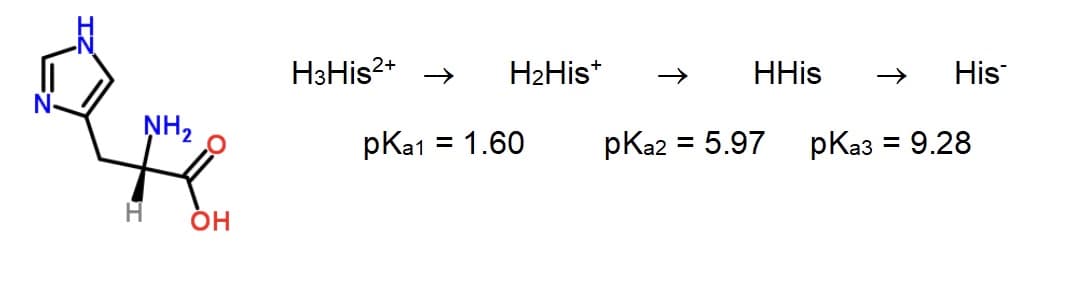
Step by step
Solved in 3 steps

- Using the Nernst equation, calculate the equilibrium potential for Ca2 and for C1 from the following sets of data: a. Given [ Ca2+ ]0=1mM,[ Ca2+ ]i=100nM, find Eca2+ b. Given [ Cl- ]0=110mM,[ Cl- ]i=100mM, find EclIn 6O2 how many oxygen (O) atoms are presentWhat is the electron transfer of C6H12O6+O2=CO2+H2O?
- Draw all possible carboxylic acids with the formula C5H10O2.Why is it 30 to 32 ATP produced for cellular respiration in Physiology? When I calculate it, I get 32.If you measured the rate of reaction at 20°C to be 1.11 x 10-5 M/s when using 0.080 M I1- and 0.040 M S2O82-. Approximately how long will the reaction take if you were to increase the temperature to 30 °C?
- If you want to produce carbohydrates containing the heavy oxygen (18O) isotope,should you water your plants with H2 18O or inject C18O2 into the air?Acetic acid is the principal ingredient in vinegar as shown; that's why it tastes sour. At equilibrium, a solution contains [CH3CO2H] = 0.0787 M and [H3 O+] = [CH3 CO2−] = 0.00118 M. What is the value of Ka for acetic acid?Calculate the two possible values of x in this equation: x2 – 11x + 24 = 0, either by using the quadratic equation developed by Abu Abd-Allah ibn Musa al-Kwarizmi, or by factoring the integer values of x. x = 24 or 1 x = 6 or 4 x = 5 or 7 D. x = 8 or 3 x = 2 or 12



