1. A pharmacist weigh 10 g of drug and dissolve in 100 mL of water. The following concentration were recorded: Concentration (mg/mL) Time(hr) 100 95 2 90 4 85 80 8. 75 10 70 12 a. Graph and look for zero order rate constant
1. A pharmacist weigh 10 g of drug and dissolve in 100 mL of water. The following concentration were recorded: Concentration (mg/mL) Time(hr) 100 95 2 90 4 85 80 8. 75 10 70 12 a. Graph and look for zero order rate constant
Chapter8: Reaction Rates And Equilibrium
Section: Chapter Questions
Problem 8.64E
Related questions
Question
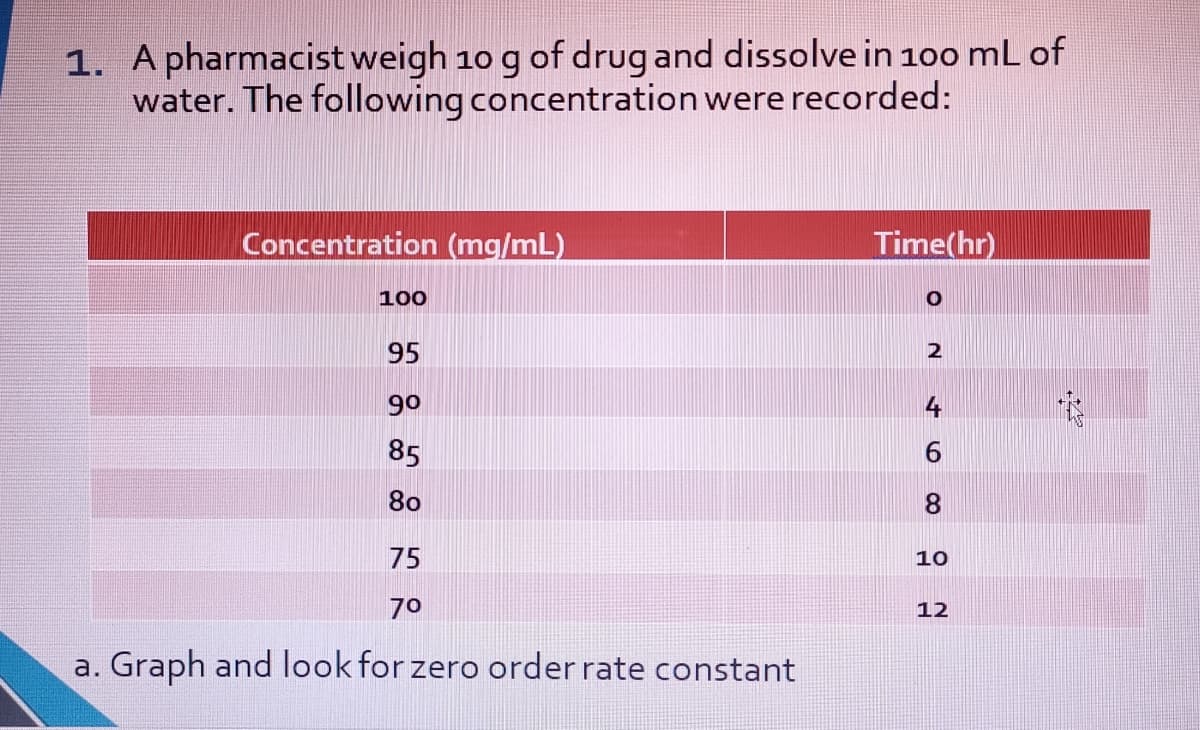
Transcribed Image Text:1. A pharmacist weigh 10 g of drug and dissolve in 100 mL of
water. The following concentration were recorded:
Concentration (mg/mL)
Time(hr)
100
95
2
90
4
85
80
8.
75
10
70
12
a. Graph and look for zero order rate constant
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning