1. Acetate buffers are used in biochemical studies of enzymes and other chemical components of cells to prevent pH changes that might change the biochemical activity of these compounds. (Ka 1.8 x 105) a) Calculate the pH of an acetate buffer that is a mixture with 0.20M acetic acid and 0.20M sodium acetate b) Calculate the pH after 2.0 mL of 0.10M NaOH is added to 100.0mL of this buffer, giving a solution with a volume 101.0m L.
1. Acetate buffers are used in biochemical studies of enzymes and other chemical components of cells to prevent pH changes that might change the biochemical activity of these compounds. (Ka 1.8 x 105) a) Calculate the pH of an acetate buffer that is a mixture with 0.20M acetic acid and 0.20M sodium acetate b) Calculate the pH after 2.0 mL of 0.10M NaOH is added to 100.0mL of this buffer, giving a solution with a volume 101.0m L.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter15: Additional Aqueous Equilibria
Section: Chapter Questions
Problem 31QRT
Related questions
Question
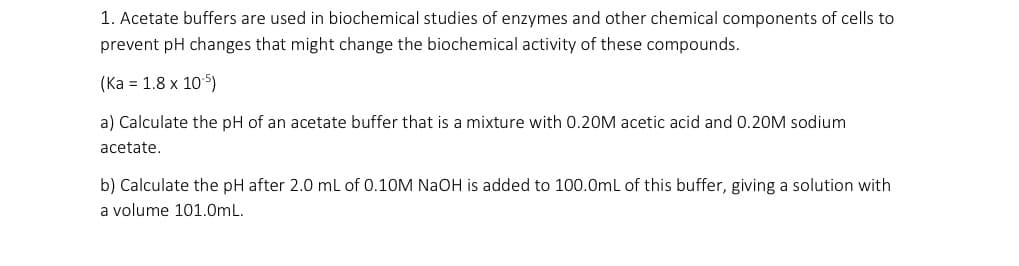
Transcribed Image Text:1. Acetate buffers are used in biochemical studies of enzymes and other chemical components of cells to
prevent pH changes that might change the biochemical activity of these compounds.
(Ka 1.8 x 105)
a) Calculate the pH of an acetate buffer that is a mixture with 0.20M acetic acid and 0.20M sodium
acetate
b) Calculate the pH after 2.0 mL of 0.10M NaOH is added to 100.0mL of this buffer, giving a solution with
a volume 101.0m L.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning