Chemistry: Form N6.4.1A Using the Combined Gas PHASES OF MATTER Page 5. Initial-1 Final-2 Volume Pressure Temperature Ti Volume Pressure Temperature VI P1 V2 P2 26.5°C 2°C K=°C+273 K=? 16.5L 107.3kPa? K=°C+273 18.0mL 104.4kPa K= ?
Chemistry: Form N6.4.1A Using the Combined Gas PHASES OF MATTER Page 5. Initial-1 Final-2 Volume Pressure Temperature Ti Volume Pressure Temperature VI P1 V2 P2 26.5°C 2°C K=°C+273 K=? 16.5L 107.3kPa? K=°C+273 18.0mL 104.4kPa K= ?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter11: Liquids And Solids
Section: Chapter Questions
Problem 11.1QE
Related questions
Question
Use the combined gas law by plugging in the numbers into it and solve the missing part
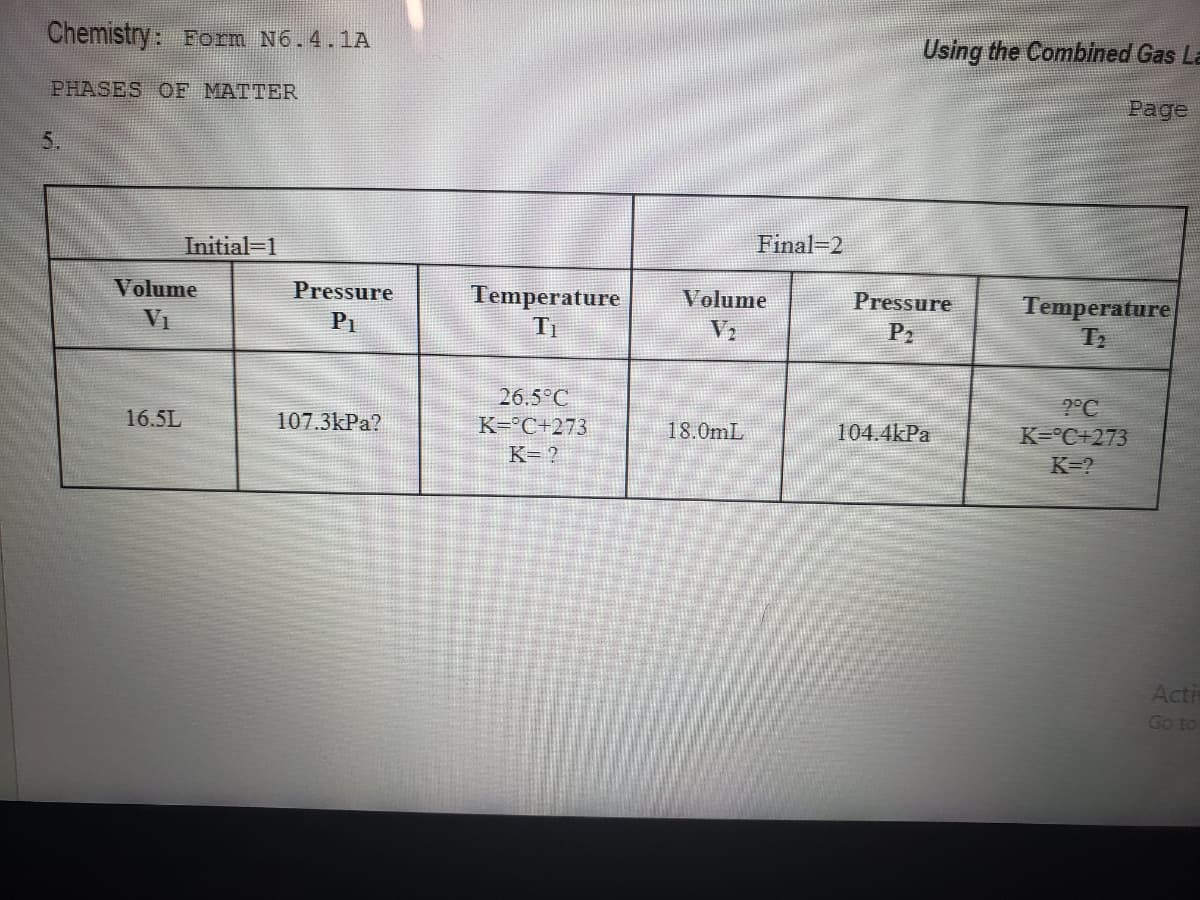
Transcribed Image Text:Chemistry: Form N6.4.1A
Using the Combined Gas La
PHASES OF MATTER
Page
5.
Initial-1
Final-2
Volume
Pressure
Temperature
Volume
Pressure
Temperature
VI
P1
V2
P2
26.5°C
2°C
16.5L
107.3kPa?
K=°C+273
18.0mL
104.4kPa
K=°C+273
K= ?
K=?
Acti
Go to
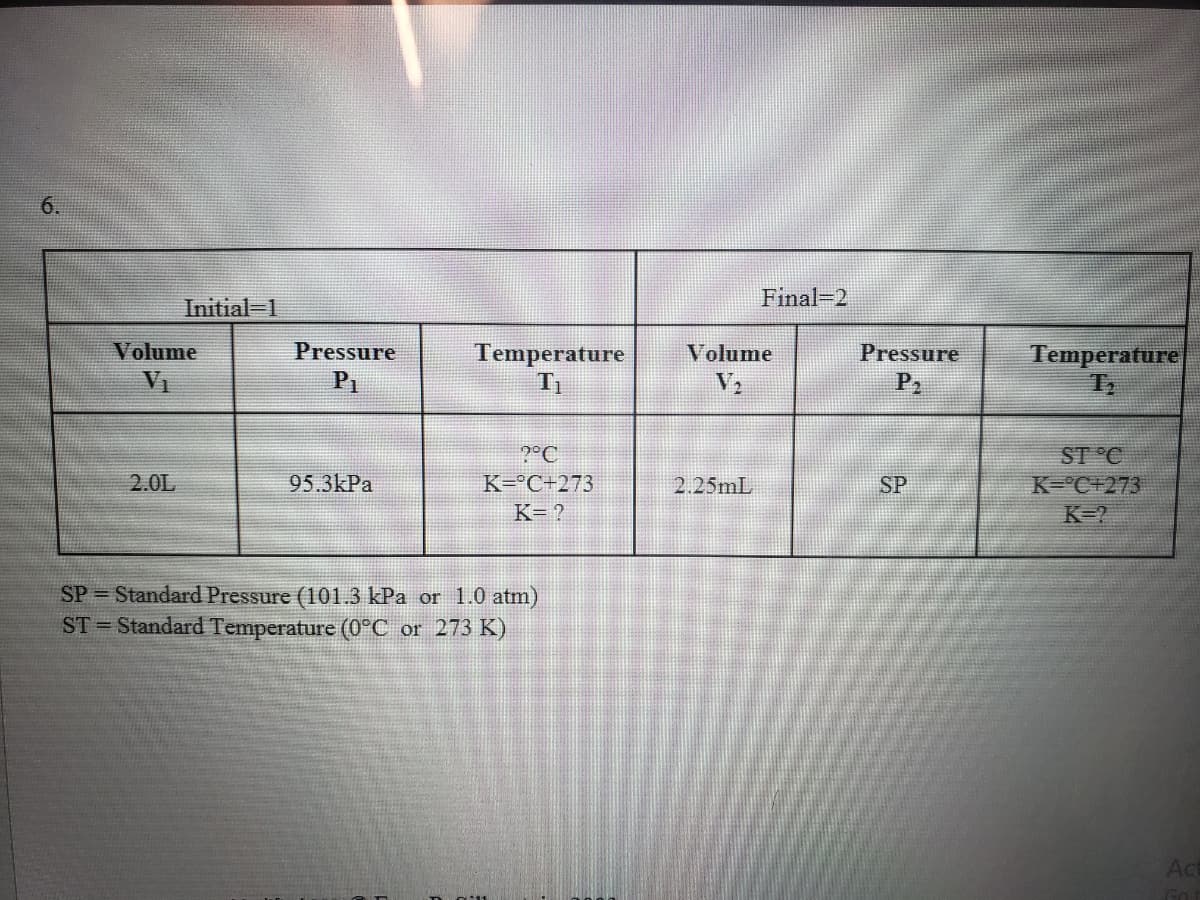
Transcribed Image Text:6.
Final-2
Initial-1
Pressure
P2
Pressure
Temperature
T2
Volume
Temperature
Volume
VI
P1
ST °C
K-°C+273
K=?
?°C
2.0L
95.3kPa
K=°C+273
2.25mL
SP
K= ?
SP = Standard Pressure (101.3 kPa or 1.0 atm)
ST = Standard Temperature (0°C or 273 K)
Act
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning