1. Dissolve A. Causes more gaseous solvent to be forced into a liquid. B. Increases solubility by moving the molecules around faster. 2. Pressure C. Increases solubility by allowing the solvent to touch more sides of the solute. 3. Crushing 4. Heating D. Expands the solvent a bit to allow more room for the solute. 5. Stirring E. When something seems to disappear inside a solvent. Polar or Non-polar? Cooking oil Salt Soluble in water Ionic Compounds
1. Dissolve A. Causes more gaseous solvent to be forced into a liquid. B. Increases solubility by moving the molecules around faster. 2. Pressure C. Increases solubility by allowing the solvent to touch more sides of the solute. 3. Crushing 4. Heating D. Expands the solvent a bit to allow more room for the solute. 5. Stirring E. When something seems to disappear inside a solvent. Polar or Non-polar? Cooking oil Salt Soluble in water Ionic Compounds
Chapter80: Crystallization: Purification Of Solids
Section: Chapter Questions
Problem 1P
Related questions
Question
100%
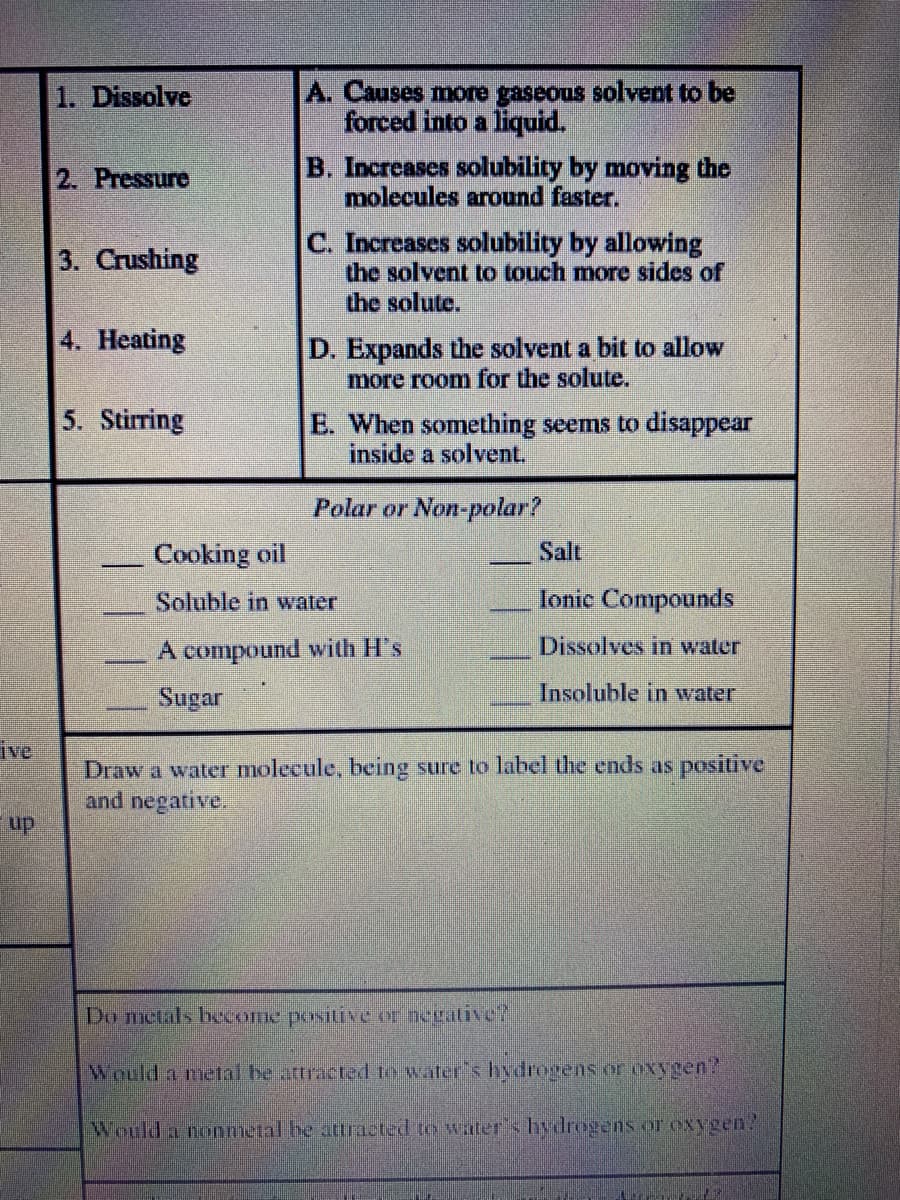
Transcribed Image Text:A. Causes more gaseous solvent to be
forced into a liquid.
1. Dissolve
B. Increases solubility by moving the
molecules around faster.
2. Pressure
C. Increases solubility by allowing
the solvent to touch more sides of
the solute.
3. Crushing
4. Heating
D. Expands the solvent a bit to allow
more room for the solute.
5. Stirring
E. When something seems to disappear
inside a solvent.
Polar or Non-polar?
Cooking oil
Salt
Soluble in water
Ionic Compounds
A compound with H's
Dissolves in water
Sugar
Insoluble in water
ive
Draw a water molecule, being sure to label the ends as positive
and negative.
up
Do metals beome positive er negalive?
Would a meal be attracted to water's hydrogens or oxygen?
Would a nonmetal be attracted to water's hydrogens or oxygen?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning