Part II, Determination of the Molar Mass of an Acid Unknown # Run Number 1 3 4 Mass of test tube + sample 1.6153g 1-5760g Mass of test tube sample 1.5760g 1,767 Mass of sample 0.0998g 0.0993g 0.1003 NaOH buret: Final reading 20.10mL 10.eomL 20.20mL 20.26mL NaOH buret: Initial reading 0.10mL O.60ML 0.10ML 0.25mh Volume of NaOH used
Part II, Determination of the Molar Mass of an Acid Unknown # Run Number 1 3 4 Mass of test tube + sample 1.6153g 1-5760g Mass of test tube sample 1.5760g 1,767 Mass of sample 0.0998g 0.0993g 0.1003 NaOH buret: Final reading 20.10mL 10.eomL 20.20mL 20.26mL NaOH buret: Initial reading 0.10mL O.60ML 0.10ML 0.25mh Volume of NaOH used
Chapter9: Acids, Bases, And Salts
Section: Chapter Questions
Problem 9.83E
Related questions
Question
Calculate the volume of NaOH used in each trial. Use sig figs.
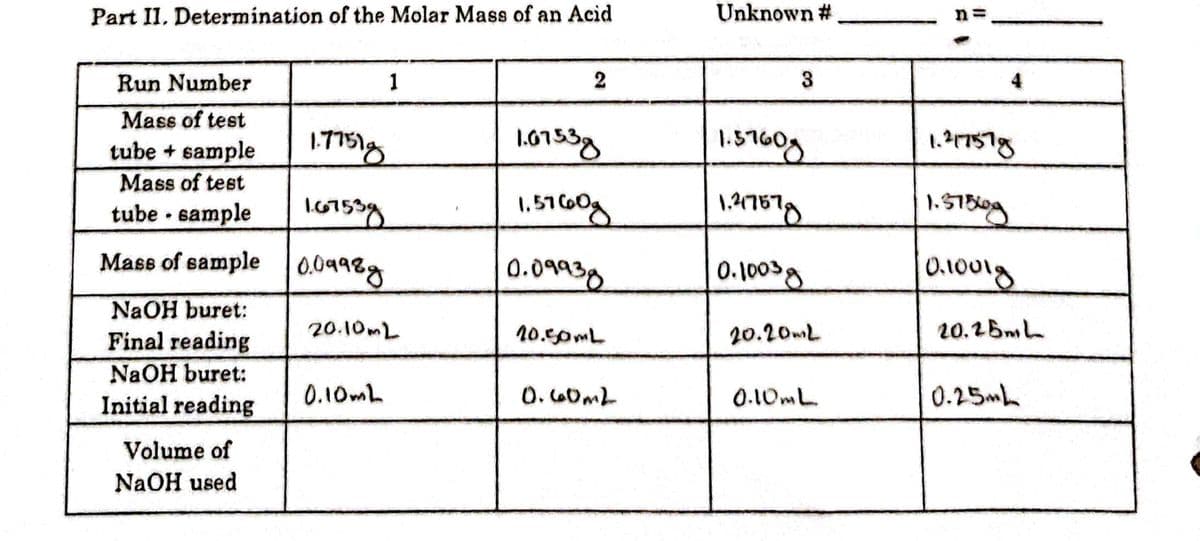
Transcribed Image Text:Part II, Determination of the Molar Mass of an Acid
Unknown #
n =
Run Number
1
2
3
4
Mass of test
tube + sample
Mass of test
16753y
Mass of sample 0.0498g
tube · sample
0.09938
0.100g
NaOH buret:
Final reading
20.10ML
20.50mL
20.20mL
20.26mL
NaOH buret:
Initial reading
0.10mL
O.60ML
0.10ML
0.25mh
Volume of
NaOH used
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning