1. Recall the following relationship developed in lecture: ƏP - P ƏT a) What is the name of this relationship? b) Evaluate for an ideal gas. c) What is the significance of this result in terms of an ideal gas? d) Evaluate for a van der Waals gas. e) What is the significance of this result in terms of a van der Waals gas?
1. Recall the following relationship developed in lecture: ƏP - P ƏT a) What is the name of this relationship? b) Evaluate for an ideal gas. c) What is the significance of this result in terms of an ideal gas? d) Evaluate for a van der Waals gas. e) What is the significance of this result in terms of a van der Waals gas?
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.52E: Under what conditions of volume does a van der Waals gas behave like an ideal gas? Use the van der...
Related questions
Question
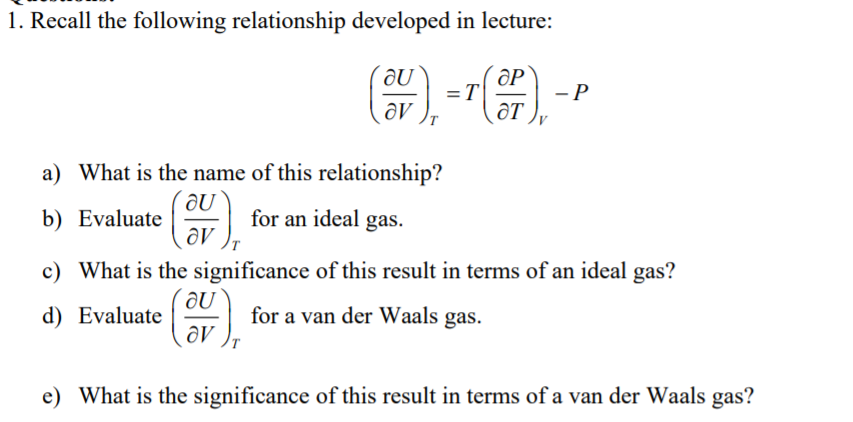
Transcribed Image Text:1. Recall the following relationship developed in lecture:
ƏP
- P
ƏT
a) What is the name of this relationship?
b) Evaluate
for an ideal gas.
c) What is the significance of this result in terms of an ideal gas?
d) Evaluate
for a van der Waals gas.
e) What is the significance of this result in terms of a van der Waals gas?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax