1. This experiment is a multistep synthesis. In the first step as described in the textbook, acetanilide (1) reacts with chlorosulfonic acid to give p-acetamidobenzenesulfonyl chloride (2). HN HO-S-CI HN O=$=O ČI 1. To obtain pure (2), it is necessary to destroy the excess chlorosulfonic acid with water. Write the equation showing how chlorosulfonic acid reacts with water, Hint: water acts as a nucleonhile
1. This experiment is a multistep synthesis. In the first step as described in the textbook, acetanilide (1) reacts with chlorosulfonic acid to give p-acetamidobenzenesulfonyl chloride (2). HN HO-S-CI HN O=$=O ČI 1. To obtain pure (2), it is necessary to destroy the excess chlorosulfonic acid with water. Write the equation showing how chlorosulfonic acid reacts with water, Hint: water acts as a nucleonhile
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.26QAP
Related questions
Question
100%
Transcribed Image Text:s for E X
Bb Error 401
Bb Exp 51 Postlab - Sulfanilami x
M Inbox (1,713) - ranjansah94 X
obcswebdav/pid-4022217-dt-content-rid-100630455_1/courses/2212-merged-CHEM2125SEC101-CHEM.
(D Page view
A Read aloud
V Draw
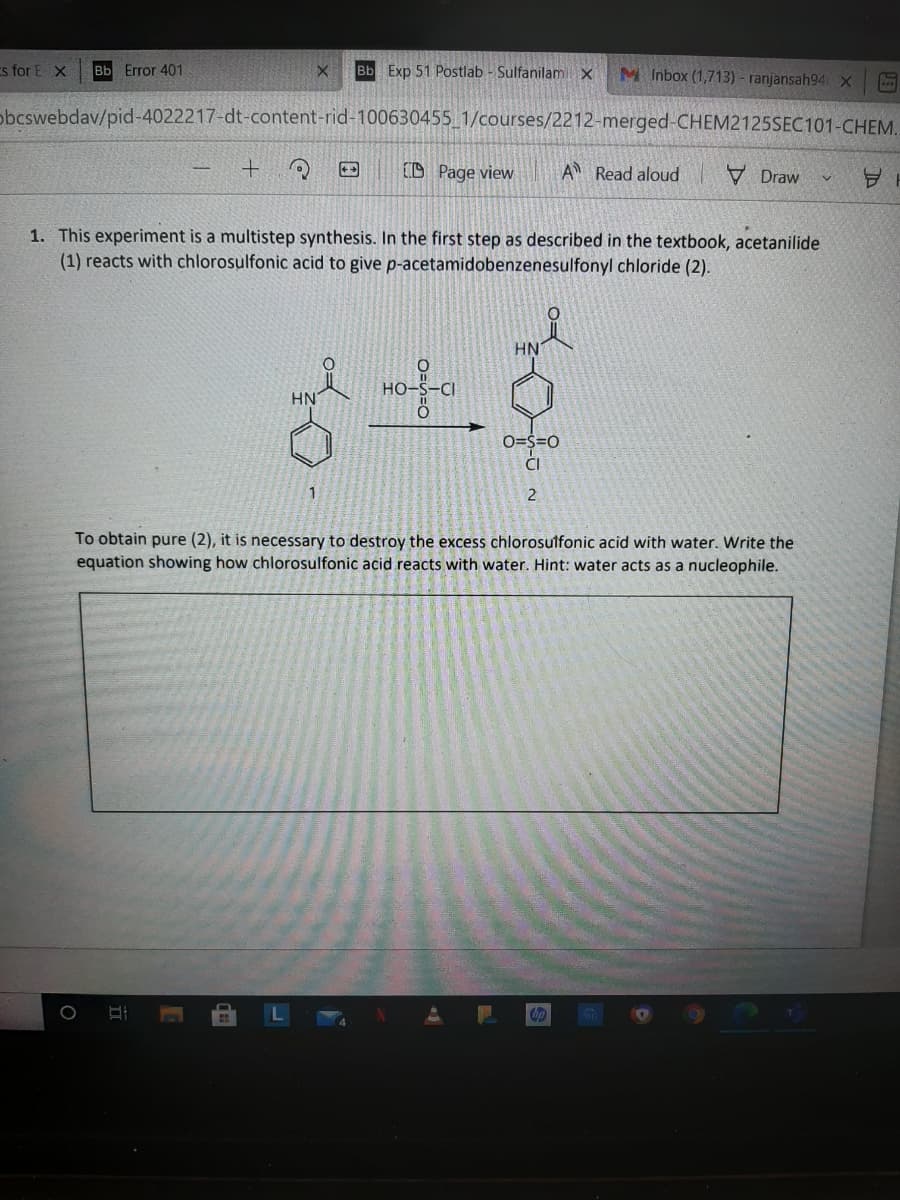
1. This experiment is a multistep synthesis. In the first step as described in the textbook, acetanilide
(1) reacts with chlorosulfonic acid to give p-acetamidobenzenesulfonyl chloride (2).
HN
HO
HN
O=S=0
ČI
2
To obtain pure (2), it is necessary to destroy the excess chlorosulfonic acid with water. Write the
equation showing how chlorosulfonic acid reacts with water. Hint: water acts as a nucleophile.
hp
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning