1.The reduction of butanoic acid can form butanal and butan-1-ol. The boiling points of these compounds are shown in Table 1 below. Table 1-Boiling points of butanyl- compounds. Boiling Point (°C) Compound butan 1-ol 120 butanal 75 butanoic acid 164 a) Write a balanced chemical equation for this reaction. Clearly indicate what conditions, catalysts, solvents, or other reagents would be required to perform this reaction. Include state symbols. b) Explain, with reference to intermolecular forces, how the process of distillation could be used to separate butan-1-ol from the other organic compounds in this reaction mixture. c) Describe an experimental procedure for a simple test that can be performed to confirm that the sample of butan-1-ol obtained by distillation does not contain any butanoic acid.
1.The reduction of butanoic acid can form butanal and butan-1-ol. The boiling points of these compounds are shown in Table 1 below. Table 1-Boiling points of butanyl- compounds. Boiling Point (°C) Compound butan 1-ol 120 butanal 75 butanoic acid 164 a) Write a balanced chemical equation for this reaction. Clearly indicate what conditions, catalysts, solvents, or other reagents would be required to perform this reaction. Include state symbols. b) Explain, with reference to intermolecular forces, how the process of distillation could be used to separate butan-1-ol from the other organic compounds in this reaction mixture. c) Describe an experimental procedure for a simple test that can be performed to confirm that the sample of butan-1-ol obtained by distillation does not contain any butanoic acid.
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
Chapter20: Acidity And Pka Of Phenols
Section: Chapter Questions
Problem 11E
Related questions
Question
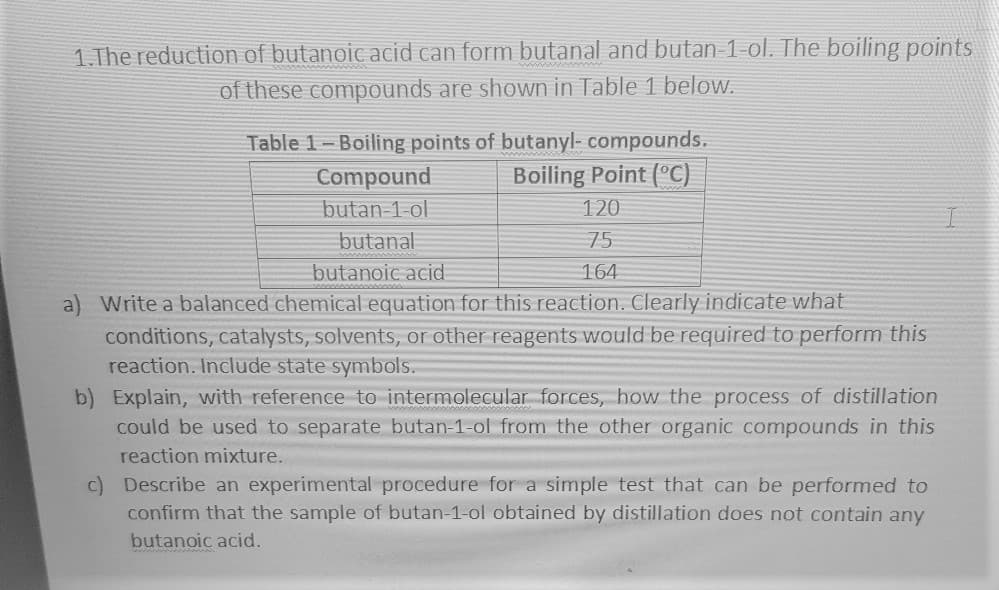
Transcribed Image Text:1.The reduction of butanoic acid can form butanal and butan-1-ol. The boiling points
of these compounds are shown in Table 1 below.
Table 1-Boiling points of butanyl- compounds.
Boiling Point (°C)
Compound
butan 1-ol
120
butanal
75
butanoic acid
164
a) Write a balanced chemical equation for this reaction.. Clearly indicate what
conditions, catalysts, solvents, or other reagents would be required to perform this
reaction. Include state symbols.
b) Explain, with reference to intermolecular forces, how the process of distillation
could be used to separate butan-1-ol from the other organic compounds in this
reaction mixture.
c) Describe an experimental procedure for a simple test that can be performed to
confirm that the sample of butan-1-ol obtained by distillation does not contain any
butanoic acid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning
