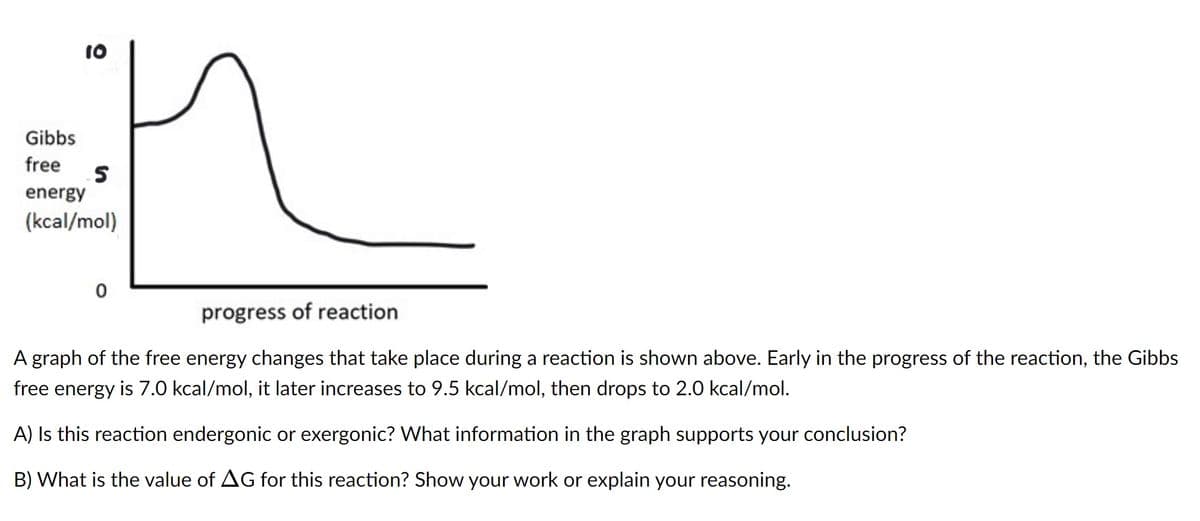
10 n 0 progress of reaction A graph of the free energy changes that take place during a reaction is shown above. Early in the progress of the reaction, the Gibbs free energy is 7.0 kcal/mol, it later increases to 9.5 kcal/mol, then drops to 2.0 kcal/mol. A) Is this reaction endergonic or exergonic? What information in the graph supports your conclusion? B) What is the value of AG for this reaction? Show your work or explain your reasoning. Gibbs free energy (kcal/mol)
10 n 0 progress of reaction A graph of the free energy changes that take place during a reaction is shown above. Early in the progress of the reaction, the Gibbs free energy is 7.0 kcal/mol, it later increases to 9.5 kcal/mol, then drops to 2.0 kcal/mol. A) Is this reaction endergonic or exergonic? What information in the graph supports your conclusion? B) What is the value of AG for this reaction? Show your work or explain your reasoning. Gibbs free energy (kcal/mol)
Biochemistry
9th Edition
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Chapter15: The Importance Of Energy Changes And Electron Transfer In Metabolism
Section: Chapter Questions
Problem 12RE: MATHEMATICAL Consider the reaction AB+C, where G=0.00. (a) What is the value of G (not G) when the...
Related questions
Question
100%
Transcribed Image Text:10
n
progress of reaction
A graph of the free energy changes that take place during a reaction is shown above. Early in the progress of the reaction, the Gibbs
free energy is 7.0 kcal/mol, it later increases to 9.5 kcal/mol, then drops to 2.0 kcal/mol.
A) Is this reaction endergonic or exergonic? What information in the graph supports your conclusion?
B) What is the value of AG for this reaction? Show your work or explain your reasoning.
Gibbs
free
energy
(kcal/mol)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Biology 2e
Biology
ISBN:
9781947172517
Author:
Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:
OpenStax