10.57 The metabolic oxidation of glucose, C6H1206, in our bodies produces CO2, which is expelled from our lungs as a gas: COH12O6(aq) + 6 O2(g) 6 CO2(g) + 6 H20(1) (a) Calculate the volume of dry CO2 produced at body temperature (37 °C) and 0.970 atm when 24.5 g of glucose is consumed in this reaction. (b) Calculate the volume of Oxygen you would need, at 1.00 atm and 298 K, to completely oxidize 50.0 g of glucose.
10.57 The metabolic oxidation of glucose, C6H1206, in our bodies produces CO2, which is expelled from our lungs as a gas: COH12O6(aq) + 6 O2(g) 6 CO2(g) + 6 H20(1) (a) Calculate the volume of dry CO2 produced at body temperature (37 °C) and 0.970 atm when 24.5 g of glucose is consumed in this reaction. (b) Calculate the volume of Oxygen you would need, at 1.00 atm and 298 K, to completely oxidize 50.0 g of glucose.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter5: Gases
Section: Chapter Questions
Problem 5.59PAE: 59 During a collision, automobile air bags are inflated by the N2 gas formed by the explosive...
Related questions
Question
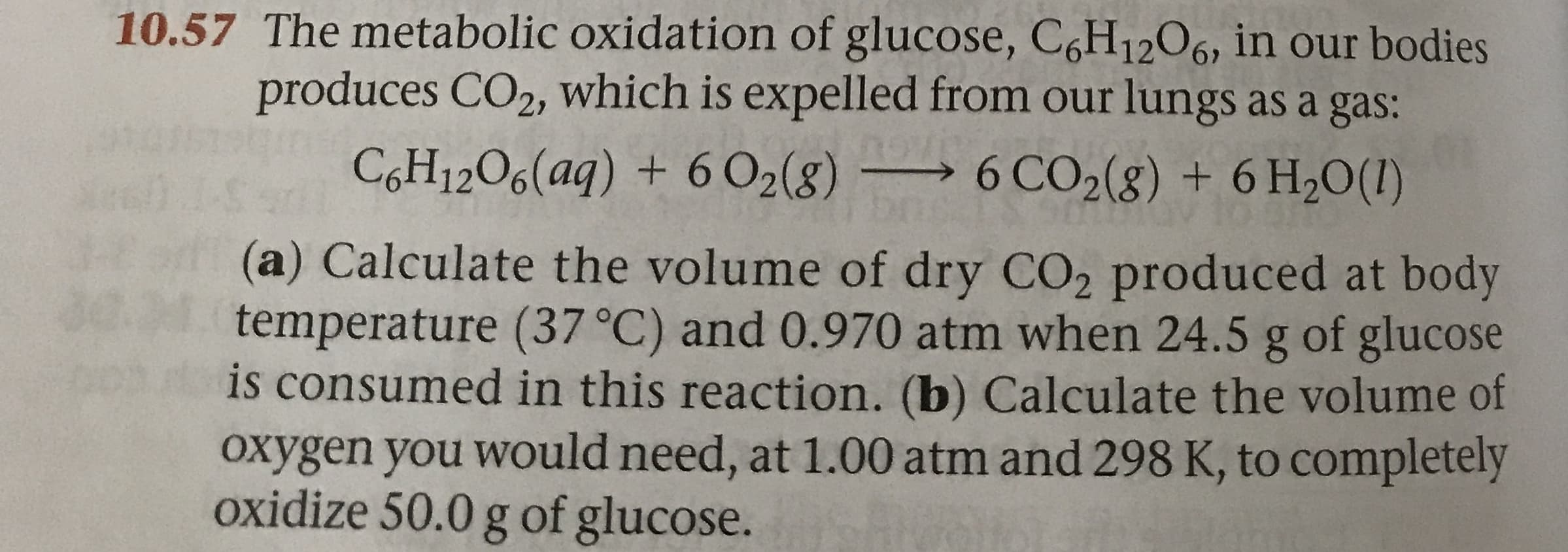
Transcribed Image Text:10.57 The metabolic oxidation of glucose, C6H1206, in our bodies
produces CO2, which is expelled from our lungs as a gas:
COH12O6(aq) + 6 O2(g) 6 CO2(g) + 6 H20(1)
(a) Calculate the volume of dry CO2 produced at body
temperature (37 °C) and 0.970 atm when 24.5 g of glucose
is consumed in this reaction. (b) Calculate the volume of
Oxygen you would need, at 1.00 atm and 298 K, to completely
oxidize 50.0 g of glucose.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning