Groups are from left to right Moet Reactive Group Loast Roactive Group The first group has electron and one energy level -14 He 2A IS- Be Ne 12 14 Increase number of valence electrons as you go down the PT Na 12 38 27 Co 20 Ca Sc TI Cr NI Cu Zn Ga Ge As Kr 43 To 44 Zr Mo 45 Rh Rb Sr Nb Ru Pd Ag Cd In Sn Sb Te Xe 11.7 72 74 75 77 Ir 70 Cs Ba Lu Ta Re Os Au Hg Pb Po At Rn BI Cammin 117 104 Rt 105 Db 115 Me 103 106 109 110 111 112 113 114 118 Fr Ra Lr Bh Hs Mt Ds Rg Cn Nh Lv Ts Og Aunicm 70 Lanthanide Series La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb 140M 1401 14424 100 Fm 102 90 Th 04 Pu 101 Actinide Series Ac Pa Am Cm Bk Es Md No Sae enational on of Pand Appled Chemiy Elements in the same period have the same number of valence electrons and similar properties PERIO09 (RE
Groups are from left to right Moet Reactive Group Loast Roactive Group The first group has electron and one energy level -14 He 2A IS- Be Ne 12 14 Increase number of valence electrons as you go down the PT Na 12 38 27 Co 20 Ca Sc TI Cr NI Cu Zn Ga Ge As Kr 43 To 44 Zr Mo 45 Rh Rb Sr Nb Ru Pd Ag Cd In Sn Sb Te Xe 11.7 72 74 75 77 Ir 70 Cs Ba Lu Ta Re Os Au Hg Pb Po At Rn BI Cammin 117 104 Rt 105 Db 115 Me 103 106 109 110 111 112 113 114 118 Fr Ra Lr Bh Hs Mt Ds Rg Cn Nh Lv Ts Og Aunicm 70 Lanthanide Series La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb 140M 1401 14424 100 Fm 102 90 Th 04 Pu 101 Actinide Series Ac Pa Am Cm Bk Es Md No Sae enational on of Pand Appled Chemiy Elements in the same period have the same number of valence electrons and similar properties PERIO09 (RE
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 30P: The molecular ion HeH+ has an equilibrium bond length of 0.774 Å. Draw an electron correlation...
Related questions
Question
Circle this periodic table images to indicate which information is incorrect
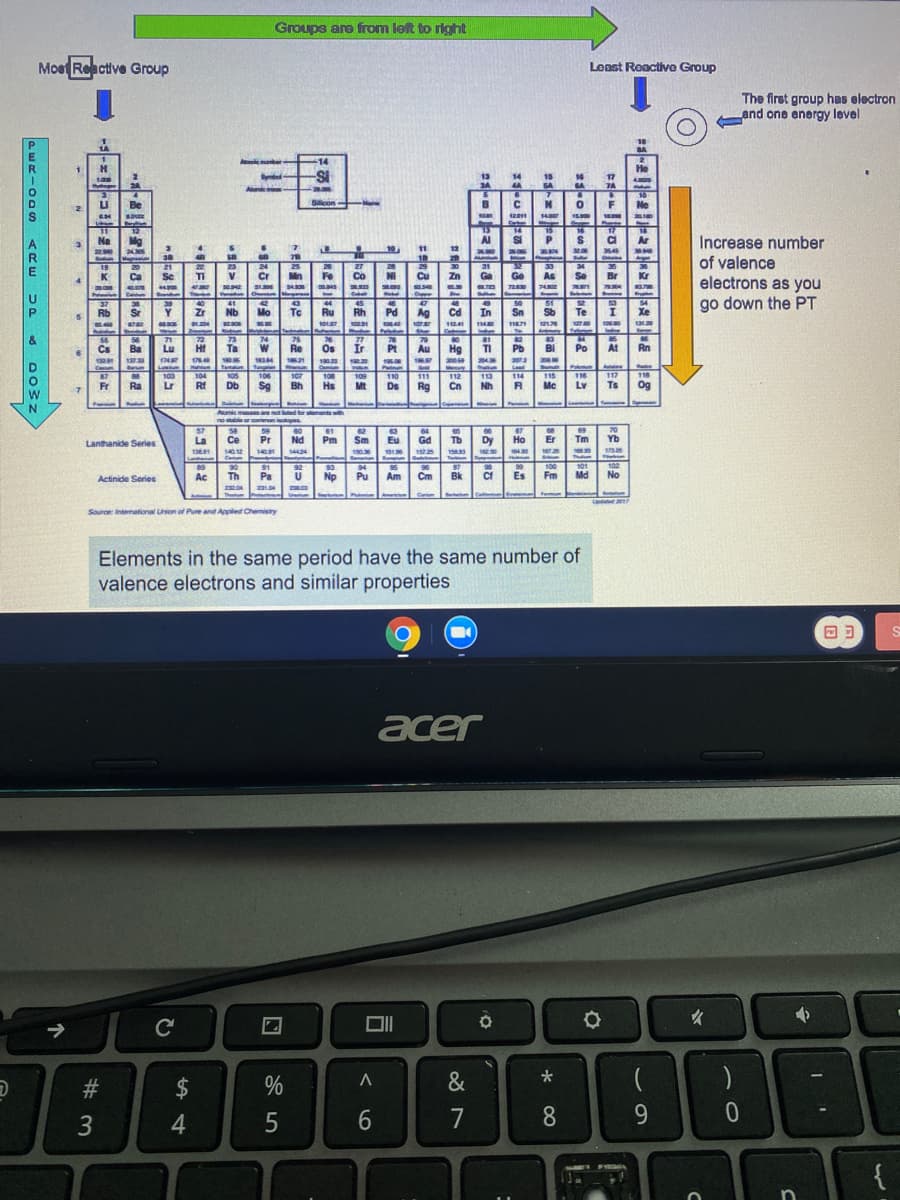
Transcribed Image Text:Groups are from left to right
Moet Reactive Group
Least Reactive Group
The first group has electron
and one energy level
-14
H
He
13
3A
14
15
17
7A
IS-
2A
An -
10
Be
Silicon
Ne
san
12
14
15
16
17
18
Ar
Increase number
Na
Mg
Al
SI
3
220
24305
10
11
12
30.00
38
78
of valence
19
K
20
21
23
24
25
26
27
28
30
31
33
34
36
Ca
Sc
TI
V
Cr
Mn
Fe
Co
NI
Cu
Zn
Ga
Ge
As
Se
Br
Kr
electrons as you
74.82
O
U
C
37
43
Te
44
Ru
go down the PT
40
41
45
46
47
50
51
53
54
Rb
Sr
Zr
Nb
Mo
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
Xe
112
12178
Sher
C
Tmen
&
56
Ba
74
75
Re
71
72
Hf
76
77
78
81
82
Cs
Lu
Ta
Os
Ir
Pt
Au
Hg
TI
Pb
BI
Po
At
Rn
180
Tataln
105
Db
186.21
20
20
20
190.
Cam
108
Hs
D.
Punn
Pakum
C
87
Fr
104
Rt
109
Mt
114
115
A
111
117
118
103
Lr
106
107
110
112
113
116
Ra
Sg
Bh
Ds
Rg
Cn
Nh
Mc
Lv
Ts
Og
Aumicmeseaeted ement
wth
noeor enen
57
La
62
64
67
Lanthanide Series
Ce
Pr
Nd
Pm
Sm
Eu
Gd
Tb
Dy
Но
Er
Tm
Yb
140.12
Ce
90
Th
1491
14424
1503
Tem
91
92
93
94
Pu
101
102
89
Ac
96
90
90
100
Pa
U
Np
Am
Cm
Bk
Cf
Es
Fm
Md
No
Actinide Series
Cartm
Souroe: Intenational Union of Pure and Appled Chemisity
Elements in the same period have the same number of
valence electrons and similar properties
acer
C
#3
$
&
4
7
8
9.
{
个
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax