2) For a face-centered cube, show the calculation to determine the number of atoms per cell and the total number of atoms in 6 unit cells. Page 541
2) For a face-centered cube, show the calculation to determine the number of atoms per cell and the total number of atoms in 6 unit cells. Page 541
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter11: States Of Matter; Liquids And Solids
Section: Chapter Questions
Problem 11.113QP
Related questions
Question
Please show step-by-step solution.

Transcribed Image Text:2) For a face-centered cube, show the calculation to determine the number of atoms per cell
and the total number of atoms in 6 unit cells. Page 541
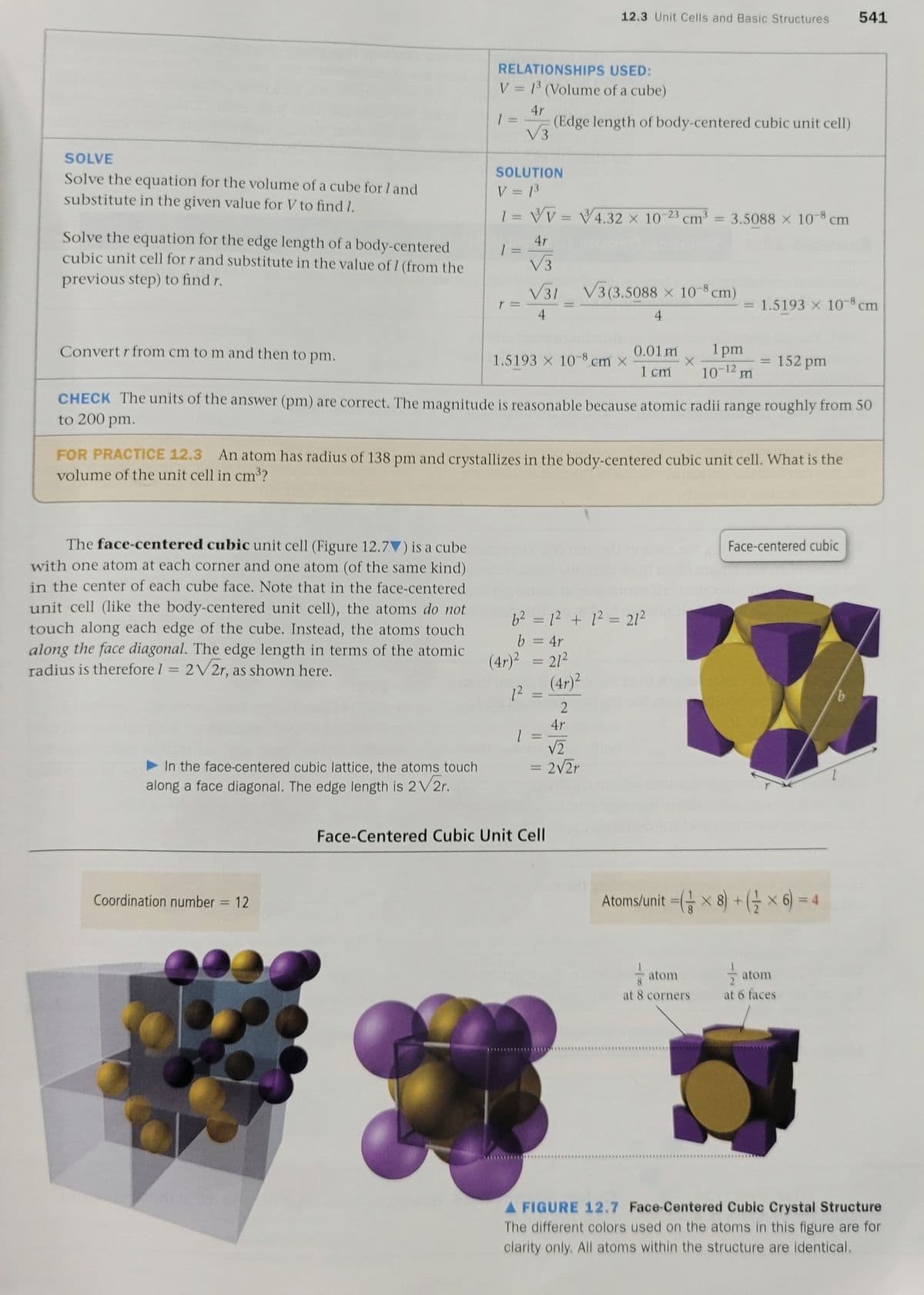
Transcribed Image Text:12.3 Unit Cells and Basic Structures
541
RELATIONSHIPS USED:
V = 1³ (Volume of a cube)
4r
(Edge length of body-centered cubic unit cell)
V3
1 =
%3D
SOLVE
SOLUTION
Solve the equation for the volume of a cube for I and
substitute in the given value for V to find I.
V = 13
1 = Vv = V4.32 × 10-23 cm
3.5088 x 10-8 cm
%3D
Solve the equation for the edge length of a body-centered
cubic unit cell for r and substitute in the value of 1 (from the
4r
1 =
V3
previous step) to find r.
V31
V3 (3.5088 × 10 8 cm)
= 1.5193 x 10-8 cm
4
4
Convert r from cm to m and then to pm.
0.01 m
1 pm
1.5193 X 10-8 cm x
152 pm
%3D
1 cm
W Z1-0
CHECK The units of the answer (pm) are correct. The magnitude is reasonable because atomic radii range roughly from 50
to 200 pm.
FOR PRACTICE 12.3 An atom has radius of 138 pm and crystallizes in the body-centered cubic unit cell. What is the
volume of the unit cell in cm³?
The face-centered cubic unit cell (Figure 12.7▼) is a cube
Face-centered cubic
with one atom at each corner and one atom (of the same kind)
in the center of each cube face. Note that in the face-centered
unit cell (like the body-centered unit cell), the atoms do not
touch along each edge of the cube. Instead, the atoms touch
along the face diagonal. The edge length in terms of the atomic
radius is therefore I = 2V2r, as shown here.
62 = 12 + 1? = 21²
b = 4r
(4r)2 = 212
(4r)2
%3D
%3D
12
4r
%3D
V2
In the face-centered cubic lattice, the atoms touch
along a face diagonal. The edge length is 2V2r.
= 2V2r
Face-Centered Cubic Unit Cell
Coordination number = 12
Atoms/unit = x 8) + (G x 6) = 4
atom
atom
at 8 cornerS
at 6 faces
A FIGURE 12.7 Face-Centered Cubic Crystal Structure
The different colors used on the atoms in this figure are for
clarity only. All atoms within the structure are identical.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning