2. Give the IUPAC name for the molecule CH3CH2CH(C2H5)CH(CH;)CH3. 3. Write the mechanism for the preparation of 1, 1- dichloroethane from ethylene. 4. Monochlorodecane (C10H21CI) is used in the preparation of surfactants though not available to any appreciable extent in natural products. a. Write two reaction paths for the production of monochlorodecane (CıoH21CI) by reaction of i. Decane (C10H22) with molecular chlorine (Cl2). Decene (C10H20) with hydrogen chloride (HC). b. What type of reaction occurs in both (i) and (ii) above? ii. 5. A moth repellent, para-dichlorobenzene, has the composition 49.1 % C, 2.7 % H and 48.2 % Cl. i. Determine the empirical formula of the compound. ii. If the molecular weight of the repellent is 147 amu, what is the molecular formula? 6. 2,3 – dimethyl – 2- butanol is subjected to acid catalyzed dehydration and yields a mixture of two isomeric alkanes. i. Draw the bond line structure for this compound. Identify the two alkenes Which one is the major product on the basis of the Zaitsev rule? ii. iii.
2. Give the IUPAC name for the molecule CH3CH2CH(C2H5)CH(CH;)CH3. 3. Write the mechanism for the preparation of 1, 1- dichloroethane from ethylene. 4. Monochlorodecane (C10H21CI) is used in the preparation of surfactants though not available to any appreciable extent in natural products. a. Write two reaction paths for the production of monochlorodecane (CıoH21CI) by reaction of i. Decane (C10H22) with molecular chlorine (Cl2). Decene (C10H20) with hydrogen chloride (HC). b. What type of reaction occurs in both (i) and (ii) above? ii. 5. A moth repellent, para-dichlorobenzene, has the composition 49.1 % C, 2.7 % H and 48.2 % Cl. i. Determine the empirical formula of the compound. ii. If the molecular weight of the repellent is 147 amu, what is the molecular formula? 6. 2,3 – dimethyl – 2- butanol is subjected to acid catalyzed dehydration and yields a mixture of two isomeric alkanes. i. Draw the bond line structure for this compound. Identify the two alkenes Which one is the major product on the basis of the Zaitsev rule? ii. iii.
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter18: Radical Initiated Chlorination Of 1-chlorobutane
Section: Chapter Questions
Problem 1Q
Related questions
Question
100%
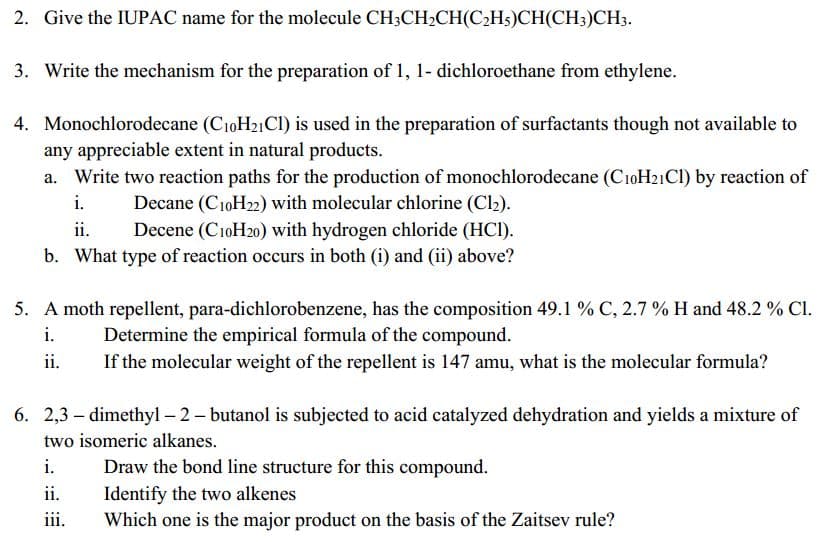
Transcribed Image Text:2. Give the IUPAC name for the molecule CH3CH2CH(C2H5)CH(CH;)CH3.
3. Write the mechanism for the preparation of 1, 1- dichloroethane from ethylene.
4. Monochlorodecane (C10H21CI) is used in the preparation of surfactants though not available to
any appreciable extent in natural products.
a. Write two reaction paths for the production of monochlorodecane (CıoH21CI) by reaction of
i.
Decane (C10H22) with molecular chlorine (Cl2).
Decene (C10H20) with hydrogen chloride (HC).
b. What type of reaction occurs in both (i) and (ii) above?
ii.
5. A moth repellent, para-dichlorobenzene, has the composition 49.1 % C, 2.7 % H and 48.2 % Cl.
i.
Determine the empirical formula of the compound.
ii.
If the molecular weight of the repellent is 147 amu, what is the molecular formula?
6. 2,3 – dimethyl – 2- butanol is subjected to acid catalyzed dehydration and yields a mixture of
two isomeric alkanes.
i.
Draw the bond line structure for this compound.
Identify the two alkenes
Which one is the major product on the basis of the Zaitsev rule?
ii.
iii.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning