2.2. Syu "pase 2110 Prac 3 Acetate 7.2 7.1 ppm 6.00 Ppm 3.0 2.5 2.0 ppm 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 ppm S60's 1.151 ° 3.319 =
2.2. Syu "pase 2110 Prac 3 Acetate 7.2 7.1 ppm 6.00 Ppm 3.0 2.5 2.0 ppm 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 ppm S60's 1.151 ° 3.319 =
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Andrei Straumanis
ChapterL3: Carbon (13c) Nmr Spectroscopy
Section: Chapter Questions
Problem 8E
Related questions
Question
Based on the HNMR spectra for 3, need help with the following question. Thank you :) (mechanism provided to show what 3)
Transcribed Image Text:b) Fully assign the provided 'H- NMR spectrum of 3
Add a labelled structure for 3
Entry
chemical shift
integration splitting
assignment
1
4
7
(add rows as needed)
3.
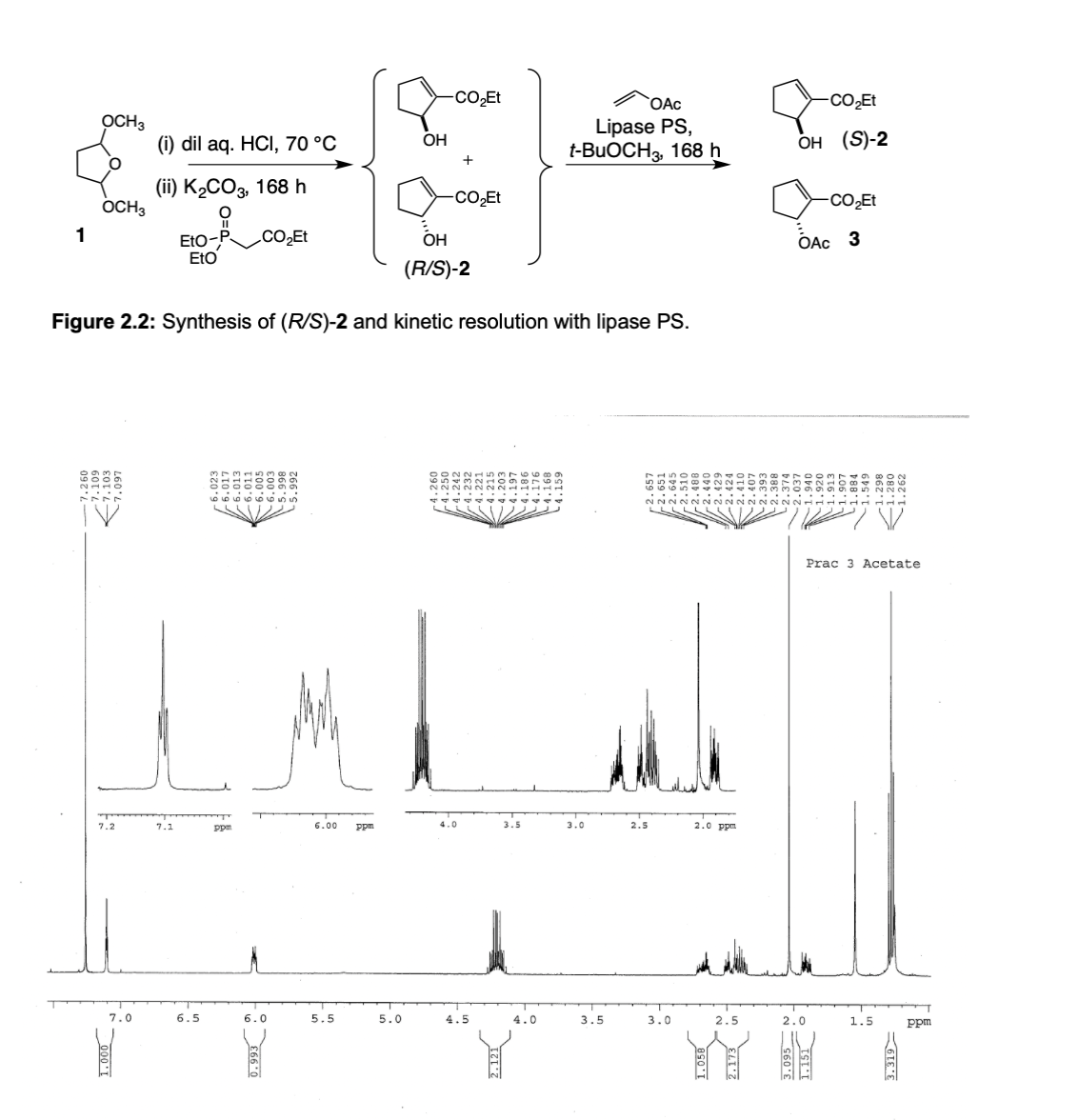
Transcribed Image Text:-CO̟ET
`OAc
-CO̟Et
OCH3
(i) dil aq. HCI, 70 °C
Lipase PS,
t-BUOCH3, 168 h
OH
он (S)-2
(ii) K2CO3, 168 h
OCH3
-CO2ET
-CO,Et
II
1
OH
Eto-P
EtO
OAc 3
(R/S)-2
Figure 2.2: Synthesis of (R/S)-2 and kinetic resolution with lipase PS.
00096
2222N T
6999 4
.... .... ... *:
1.
.... .... ...
2222N NN22
ক
LLLLL
Prac 3 Acetate
7.2
7.1
ppm
6.00
Ppm
4.0
3.5
3.0
2.5
2.0 ppm
7.0
6.5
6.0
5.5
5.0
4.5
4.0
3.5
3.0
2.5
2.0
1.5
ppm
000'I
3.095
3.319=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning