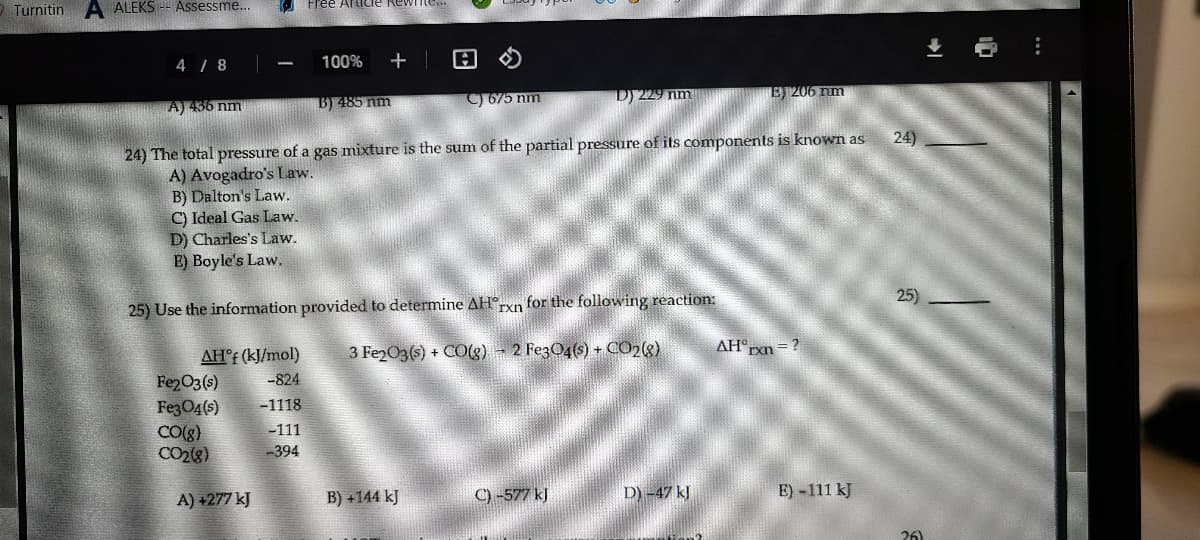
24) 24) The total pressure of a gas mixture is the sum of the partial pressure of its components is known as A) Avogadro's Law. B) Dalton's Law. C) Ideal Gas Law. D) Charles's Law. E) Boyle's Law. 25) 25) Use the information provided to determine AHxn for the following reaction: rxn 3 Fe2O3(s) + CO(g) - 2 FezO4(s) + CO2(8) AH°rxn=? AH°F (kJ/mol) Fe2O3(s) Fe3O4(s) CO(8) CO2(8) -824 -1118 -111 -394
24) 24) The total pressure of a gas mixture is the sum of the partial pressure of its components is known as A) Avogadro's Law. B) Dalton's Law. C) Ideal Gas Law. D) Charles's Law. E) Boyle's Law. 25) 25) Use the information provided to determine AHxn for the following reaction: rxn 3 Fe2O3(s) + CO(g) - 2 FezO4(s) + CO2(8) AH°rxn=? AH°F (kJ/mol) Fe2O3(s) Fe3O4(s) CO(8) CO2(8) -824 -1118 -111 -394
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.127QP: A 1.000-g sample of an unknown gas at 0C gives the following data: P(atm) V (L) 0.2500 3.1908 0.5000...
Related questions
Question
Transcribed Image Text:Turnitin
ALEKS -- Assessme...
Free Aricie RewnC.
4 / 8
100%
+ | 田の
B) 485 nm
C) 675 nm
D) 229 nm
E) 206 nm
A) 436 nm
24) The total pressure of a gas mixture is the sum of the partial pressure of its components is known as
A) Avogadro's Law.
B) Dalton's Law.
C) Ideal Gas Law.
D) Charles's Law.
E) Boyle's Law.
24)
25)
25) Use the information provided to determine AH°xn for the following reaction:
AH°F (kJ/mol)
3 Fe2O3(s) + CO(g) - 2 Fe3O4(s) + CO2(8)
AH"Exn = ?
-824
Fe2O3(s)
Fe3O4(s)
CO(g)
CO2(8)
-1118
-111
-394
A) +277 kJ
B) +144 kJ
C) -577 kJ
D)-47 kl
E)-111 kJ
26)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,