3. Below is a polypeptide with an unknown number of amino acids. (Standard 4) One the diagram below: H₂N*- Ć CH₂ I OH a. Circle EVERY peptide bond b. Circle and number each amino acid from 1, 2, 3, and so on; from left to right c. Label each amino acid as polar, non-polar, or electrically charge d. Label the N-terminus and C-terminus e. Select three pairs of amino acids that could potentially interact to influence tertiary structure AND state the bond/force/interaction type involved in each pair. || HOHHO -N-C-C-N-C-C- CH₂ | CH₂ CH₂ SH || -C- 1 CH3 || ċ-ċ-N-C- CH₂ HO CH₂ ! _ƒ_ƒ_ƒ`__ \_! CH₂ CH₂ CH₂ ΝΗ OHHO || || 1-C-C-N- C=ANH, T NH₂ | CH₂ | SH | H ||
3. Below is a polypeptide with an unknown number of amino acids. (Standard 4) One the diagram below: H₂N*- Ć CH₂ I OH a. Circle EVERY peptide bond b. Circle and number each amino acid from 1, 2, 3, and so on; from left to right c. Label each amino acid as polar, non-polar, or electrically charge d. Label the N-terminus and C-terminus e. Select three pairs of amino acids that could potentially interact to influence tertiary structure AND state the bond/force/interaction type involved in each pair. || HOHHO -N-C-C-N-C-C- CH₂ | CH₂ CH₂ SH || -C- 1 CH3 || ċ-ċ-N-C- CH₂ HO CH₂ ! _ƒ_ƒ_ƒ`__ \_! CH₂ CH₂ CH₂ ΝΗ OHHO || || 1-C-C-N- C=ANH, T NH₂ | CH₂ | SH | H ||
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
please help

Transcribed Image Text:3. Below is a polypeptide with an unknown number of amino acids. (Standard 4) One the
diagram below:
H₂N*-
I
CH₂
1
OH
a. Circle EVERY peptide bond
b. Circle and number each amino acid from 1, 2, 3, and so on; from left to right
c. Label each amino acid as polar, non-polar, or electrically charge
d. Label the N-terminus and C-terminus
e. Select three pairs of amino acids that could potentially interact to influence
tertiary structure AND state the bond/force/interaction type involved in each pair.
OHH O HHO
|| ! | ||
||
-N-C-C-N-
1
CH₂
CH₂
OH
Standard 3 Material
CH₂
I
4. For the reaction below:
SH
H
OHHOH HOHH OHH
||
|| | ||
¿
-C-N-
||
1-C-C-N-
1
CH3
CH₂
HO CH3
CH₂
|
CH₂
I
CH₂
|
NH
|
C=ANH,
NH₂
CH₂
I
SH
H
a. How many non-polar and polar bonds are in the reactants?
b. How many non-polar and polar bonds are in the products?
c. What is the enthalpy (AH) of this reaction? What is your evidence for this
response?
d. What is the entropy (AS) of this reaction? What is your evidence for this
response?
e. Is the reaction below spontaneous/non-spontaneous and endergonic/exergonic?
f. Is carbon being oxidized or reduced? Why?
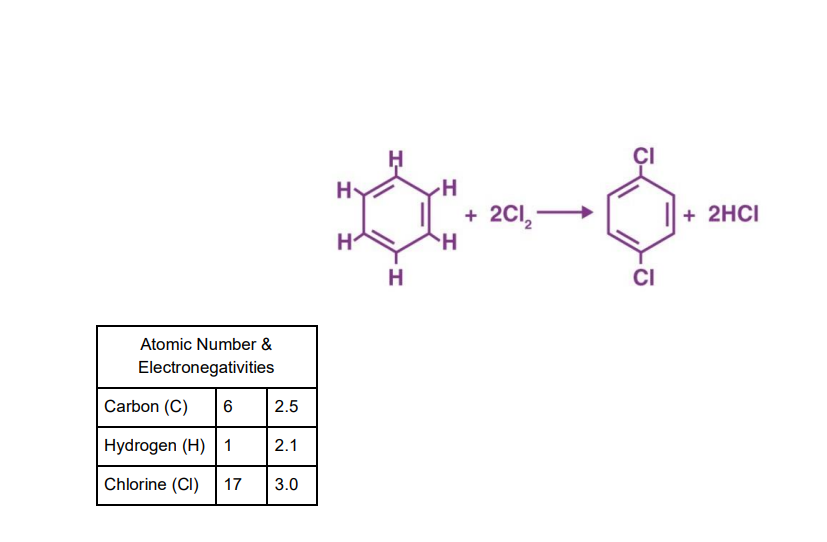
Transcribed Image Text:Atomic Number &
Electronegativities
Carbon (C) 6
Hydrogen (H) 1
Chlorine (CI) 17
2.5
2.1
3.0
H
H
D----
+ 2Cl₂
H
H
CI
H
CI
+ 2HCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 2 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON