3. Binary system acetonitrile(1)/nitromethane(2) conforms closely to Raoult's law. Vapor pressures for the pure species are given by the following Antoine equations: In P1sat/kPa=14.2724-2945.47t/°C+224.00 In P2sat/kPa=14.2043-2972.64t/°C+209.00 Prepare a Pxy diagram for a temperature of 75°C by calculating and plotting the mole fraction of acetonitrile in the vapor phase y1, and the total pressure, P, for different values of X1 as presented below: Mol fraction of acetonitrile in the liquid phase, X1 Mol fraction of acetonitrile in the liquid phase, y Total pressure, P (kPa) 0.00 0.10 0.20 0.30 0.40 0.50 0.60 0.70 0.80 0.90 1.00 Using your plot, estimate the pressure and liquid composition (X1 and X2) of this mixture when the vapor is 60-mol% acetonitrile. Using interpolation, calculate the pressure and X1 with the same given vapor composition of acetonitrile. Compare your estimation and get the % error.
3. Binary system acetonitrile(1)/nitromethane(2) conforms closely to Raoult's law. Vapor pressures for the pure species are given by the following Antoine equations: In P1sat/kPa=14.2724-2945.47t/°C+224.00 In P2sat/kPa=14.2043-2972.64t/°C+209.00 Prepare a Pxy diagram for a temperature of 75°C by calculating and plotting the mole fraction of acetonitrile in the vapor phase y1, and the total pressure, P, for different values of X1 as presented below: Mol fraction of acetonitrile in the liquid phase, X1 Mol fraction of acetonitrile in the liquid phase, y Total pressure, P (kPa) 0.00 0.10 0.20 0.30 0.40 0.50 0.60 0.70 0.80 0.90 1.00 Using your plot, estimate the pressure and liquid composition (X1 and X2) of this mixture when the vapor is 60-mol% acetonitrile. Using interpolation, calculate the pressure and X1 with the same given vapor composition of acetonitrile. Compare your estimation and get the % error.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter23: Polymeric Materials And Soft Condensed Matter
Section: Chapter Questions
Problem 14P
Related questions
Question
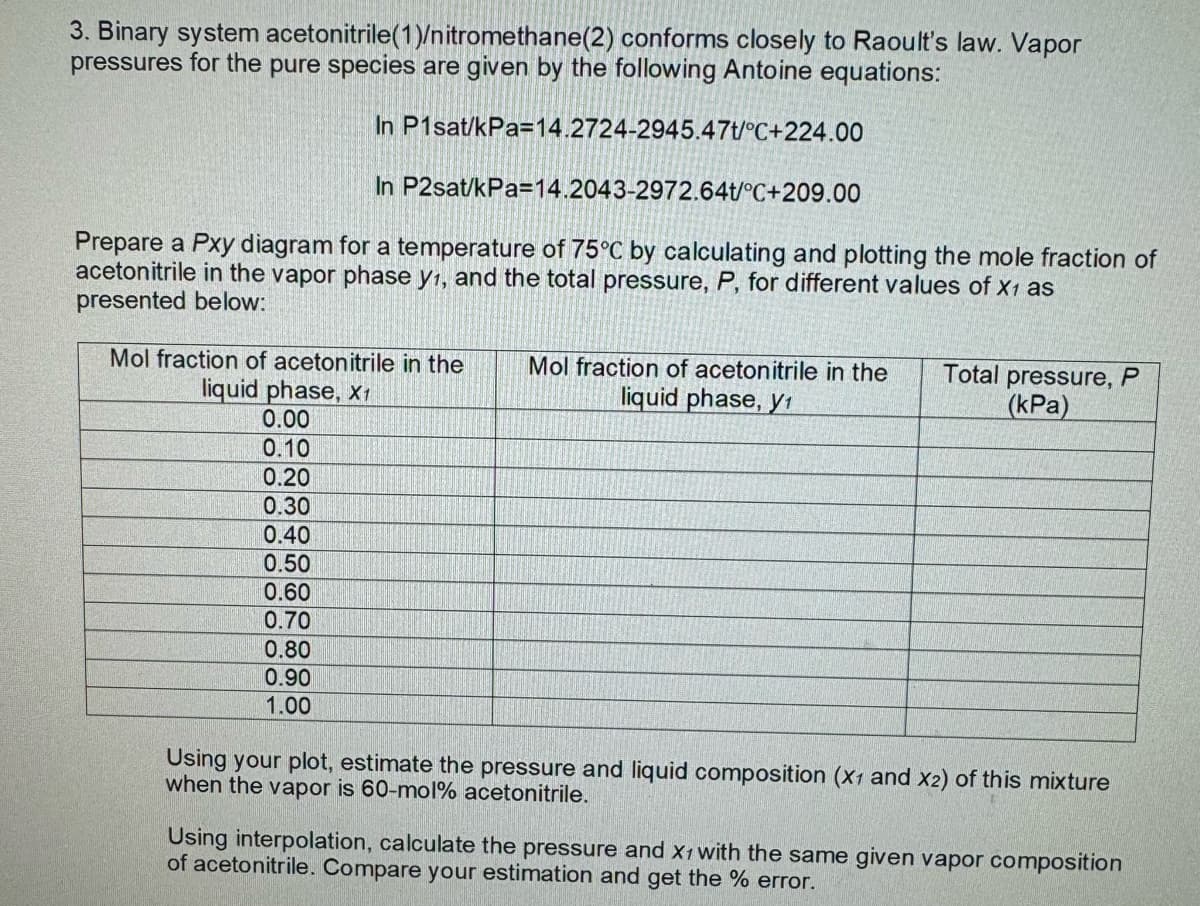
Transcribed Image Text:3. Binary system acetonitrile(1)/nitromethane(2) conforms closely to Raoult's law. Vapor
pressures for the pure species are given by the following Antoine equations:
In P1sat/kPa=14.2724-2945.47t/°C+224.00
In P2sat/kPa=14.2043-2972.64t/°C+209.00
Prepare a Pxy diagram for a temperature of 75°C by calculating and plotting the mole fraction of
acetonitrile in the vapor phase y1, and the total pressure, P, for different values of X1 as
presented below:
Mol fraction of acetonitrile in the
liquid phase, X1
Mol fraction of acetonitrile in the
liquid phase, y
Total pressure, P
(kPa)
0.00
0.10
0.20
0.30
0.40
0.50
0.60
0.70
0.80
0.90
1.00
Using your plot, estimate the pressure and liquid composition (X1 and X2) of this mixture
when the vapor is 60-mol% acetonitrile.
Using interpolation, calculate the pressure and X1 with the same given vapor composition
of acetonitrile. Compare your estimation and get the % error.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole