3. How much energy, in kilojoules, did the water lose in the experiment in # 4? (Use q = (m)(c)(AT) and then convert joules to kilojoules.) %3D
3. How much energy, in kilojoules, did the water lose in the experiment in # 4? (Use q = (m)(c)(AT) and then convert joules to kilojoules.) %3D
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter2: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 2.75E: Draw a diagram like Figure 2.11 that illustrates the change in enthalpy for the chemical reaction C...
Related questions
Question
The first page is the question and the second page is the basis.
Kindly write the explanation of your answer thanks
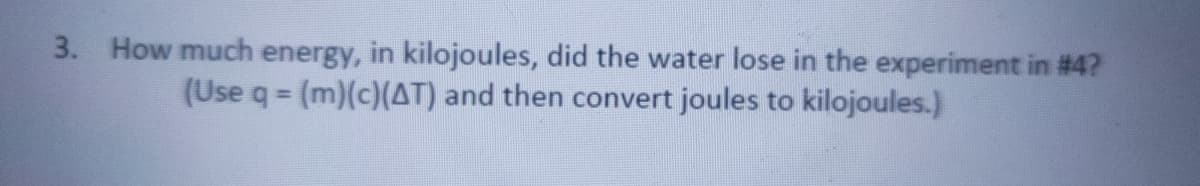
Transcribed Image Text:3. How much energy, in kilojoules, did the water lose in the experiment in #4?
(Use q (m)(c)(AT) and then convert joules to kilojoules.)
%3D

Transcribed Image Text:3) SOLUTION 20, NH4 03 (20°c) + 100g Hz0 (20° C)
šolve for
N: 2* 4 0067
a8.0134
4.C313し
209 *
: 0.25 mol
10.04476 9
49
Or 422 5 J
q. : (0 25 mol)(as.09 xl/mol) : 6.4215 *J
9:: meAT sier
502.08 Te - 10041.6
: (:20 g) (4.184 Jg°E) ( T# -20°c)
tpht 22 . 51 + 508 08 TE
10041.4 0
502 TE
10041.6 - 6 422.5
TE = 7.21° C
502
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning