3. Unknown Solution Concentration: From the calibration curve, determine the molarity of your unknown solution, not by interpolation on the graph but algebraically from the computer - generated m value in the equation for a straight line, y = mx + b. If b equals zero, then y = mx, where m is the slope of the line, x is the solution concentration and y is the measured absorbance. Since the molar absorptivity & and the path length are constants, this equation is consistent with Beer's law A = ɛbc which becomes A = mc.
3. Unknown Solution Concentration: From the calibration curve, determine the molarity of your unknown solution, not by interpolation on the graph but algebraically from the computer - generated m value in the equation for a straight line, y = mx + b. If b equals zero, then y = mx, where m is the slope of the line, x is the solution concentration and y is the measured absorbance. Since the molar absorptivity & and the path length are constants, this equation is consistent with Beer's law A = ɛbc which becomes A = mc.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter34: Particle Size Determination
Section: Chapter Questions
Problem 34.6QAP
Related questions
Question

Transcribed Image Text:3. Unknown Solution Concentration: From the calibration curve, determine the molarity of your
unknown solution, not by interpolation on the graph but algebraically from the computer-
generated m value in the equation for a straight line, y = mx + b. If b equals zero, then
y = mx, where m is the slope of the line, x is the solution concentration and y is the measured
absorbance. Since the molar absorptivity & and the path length are constants, this equation is
consistent with Beer's law A = ɛbc which becomes A = mc.
%3D
%3D
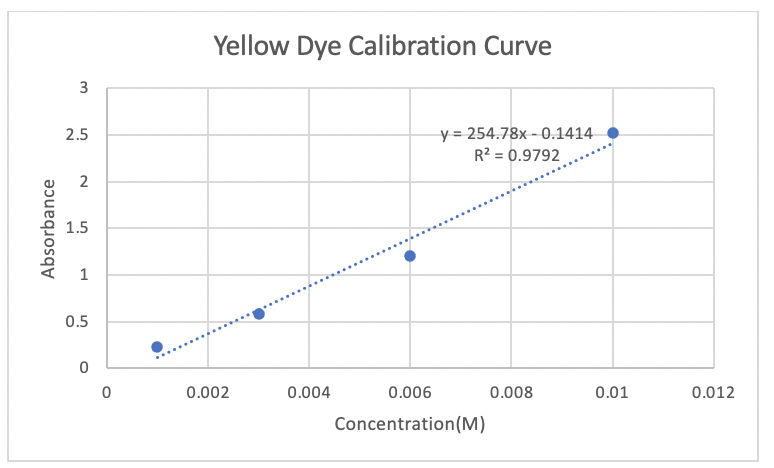
Transcribed Image Text:Yellow Dye Calibration Curve
y = 254.78x - 0.1414
R? = 0.9792
2.5
1.5
1
0.5
0.002
0.004
0.006
0.008
0.01
0.012
Concentration(M)
3.
2.
Absorbance
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
