4. Mass % NaHCO3 in Alka-Seltzer_ 5. mg of NaHCO3 per one tablet, calculated_ 6. On its label, the company claims 1904 mg NaHCO3 per tablet. Taking this to be the true value, what is the % error in the above experimental work?
4. Mass % NaHCO3 in Alka-Seltzer_ 5. mg of NaHCO3 per one tablet, calculated_ 6. On its label, the company claims 1904 mg NaHCO3 per tablet. Taking this to be the true value, what is the % error in the above experimental work?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter9: The Gaseous State
Section: Chapter Questions
Problem 34P
Related questions
Question
Both images attached go together plus please try to answer all questions

Transcribed Image Text:Calculations (continued)
3. Mass NaHCO3 used_
4. Mass % NaHCO3 in Alka-Seltzer_
5. mg of NaHCO3 per one tablet, calculated
6. On its label, the company claims 1904 mg NaHCO3 per tablet. Taking this to be the true
value, what is the % error in the above experimental work?
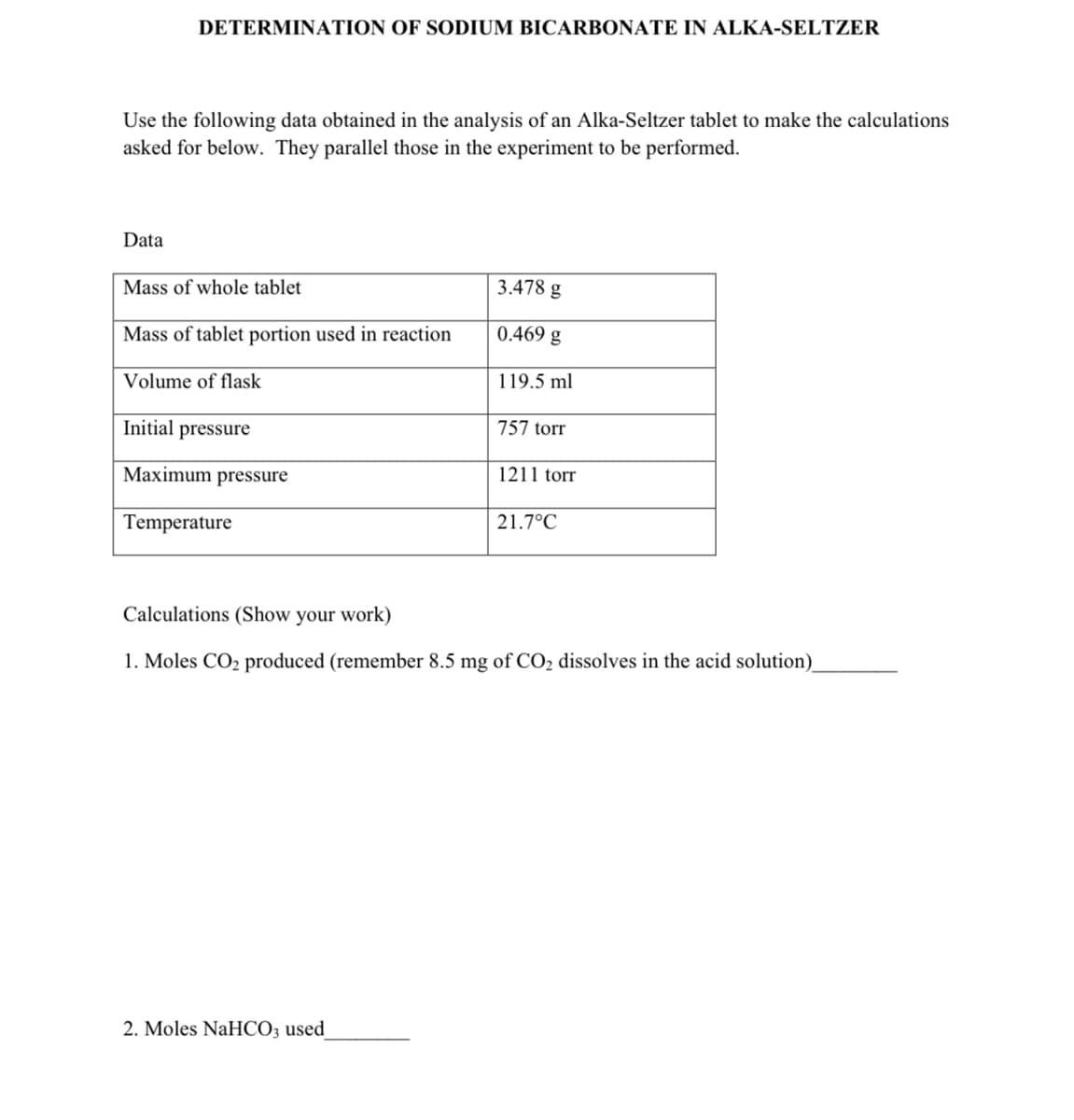
Transcribed Image Text:DETERMINATION OF SODIUM BICARBONATE IN ALKA-SELTZER
Use the following data obtained in the analysis of an Alka-Seltzer tablet to make the calculations
asked for below. They parallel those in the experiment to be performed.
Data
Mass of whole tablet
Mass of tablet portion used in reaction
Volume of flask
Initial pressure
Maximum pressure
Temperature
3.478 g
0.469 g
119.5 ml
2. Moles NaHCO3 used
757 torr
1211 torr
21.7°C
Calculations (Show your work)
1. Moles CO₂ produced (remember 8.5 mg of CO₂ dissolves in the acid solution)_
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 6 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning