4. The diagram below shows the changes in concentrations of three substances, X, Y, Z. as they take part in a chemical reaction that reaches equilibrium. All three substances are gases. Reaction Progress s78 Time (min) a) Write an equation to describe the equilibrium. b) Predict how the graph will change if more of compound Z is added at 10 min. Give reasons for your prediction.
4. The diagram below shows the changes in concentrations of three substances, X, Y, Z. as they take part in a chemical reaction that reaches equilibrium. All three substances are gases. Reaction Progress s78 Time (min) a) Write an equation to describe the equilibrium. b) Predict how the graph will change if more of compound Z is added at 10 min. Give reasons for your prediction.
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter17: Equilibrium
Section: Chapter Questions
Problem 51QAP: . For the reaction 3O2(g)2O3(g)The equilibrium constant, K, has the value 1.121054at a particular...
Related questions
Question
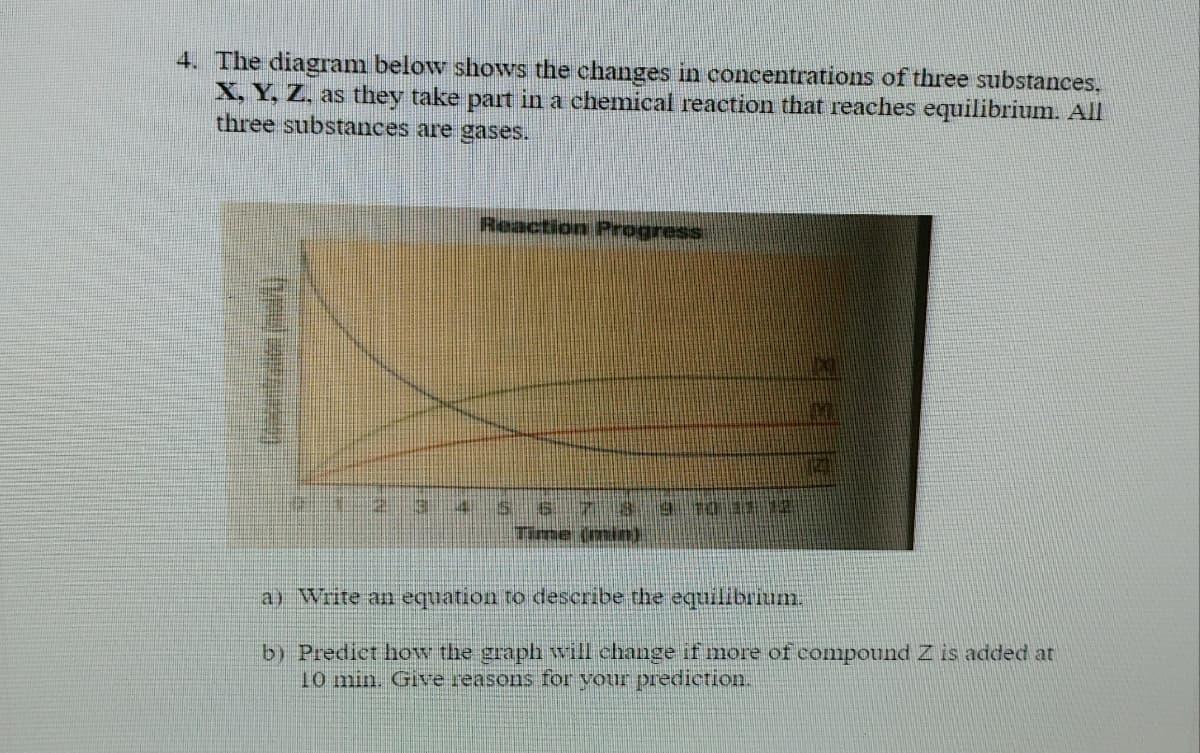
Transcribed Image Text:4. The diagram below shows the changes in concentrations of three substances,
X, Y, Z, as they take part in a chemical reaction that reaches equilibrium. All
three substances are gases.
Reaction Progress
Time (minn).
a) Write an equation to describe the equilibrium.
b) Predict how the graph will change if more of compound Z is added at
10 min. Give reasons for your prediction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning