Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter21: The Solid State: Crystals
Section: Chapter Questions
Problem 21.56E
Related questions
Question
Select the best pair of answers below justifying the reason for your answer with detailed explanations.
Answer Q49 only
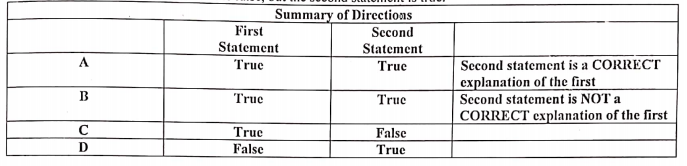
Transcribed Image Text:First
Statement
True
Summary of Directions
Second
Statement
Truc
Second statement is a CORRECT
explanation of the first
Second statement is NOT a
CORRECT explanation of the first
A.
Truc
True
C
Truc
False
True
D
False

Transcribed Image Text:FIRST STATEMENT
SECOND STATEMENT
47.
When pentanol is warmed with acidificd K:CrOr, the Cr" is orange and is reduced to Cr" is green.
colour changes from orange to green.
An azeotropic mixture can be separated by fractional For azeotropic mixtures, the liquid and vapour have the
distillation.
49.
48.
same composition.
NaCl and KCI are purely ionic compounds.
Theoretical and experimental values of lattice energies of
NaCl and KCI are very elose.
Ethanol has a higher boiling point than methoxymethane.
50.
Hydrogen bonding exists between methoxymethane
molecules
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning