5 of 24 Review Constants Periodic Table Density is an intensive physical property that relates the mass of an object to its volume. Density, which is simply the mass of an object divided by its volume, is expressed in the SI derived unit g/mL for a liquid or g/cm³ for a solid. Most substances expand or contract when heated or cooled, so the density values for a substance are temperature dependent. Part A A particular brand of gasoline has a density of 0.737 g/mL at 25 °C. How many grams of this gasoline would fill a 12.5 gal tank (1 US gal = 3.78 L)? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s)
5 of 24 Review Constants Periodic Table Density is an intensive physical property that relates the mass of an object to its volume. Density, which is simply the mass of an object divided by its volume, is expressed in the SI derived unit g/mL for a liquid or g/cm³ for a solid. Most substances expand or contract when heated or cooled, so the density values for a substance are temperature dependent. Part A A particular brand of gasoline has a density of 0.737 g/mL at 25 °C. How many grams of this gasoline would fill a 12.5 gal tank (1 US gal = 3.78 L)? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s)
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.30QAP
Related questions
Question
Transcribed Image Text:5 of 24
Review Constants Periodic Table
roperty that
s volume. Density,
ject divided by its
rived unit g/mL
Most substances
or cooled, so the
Submit
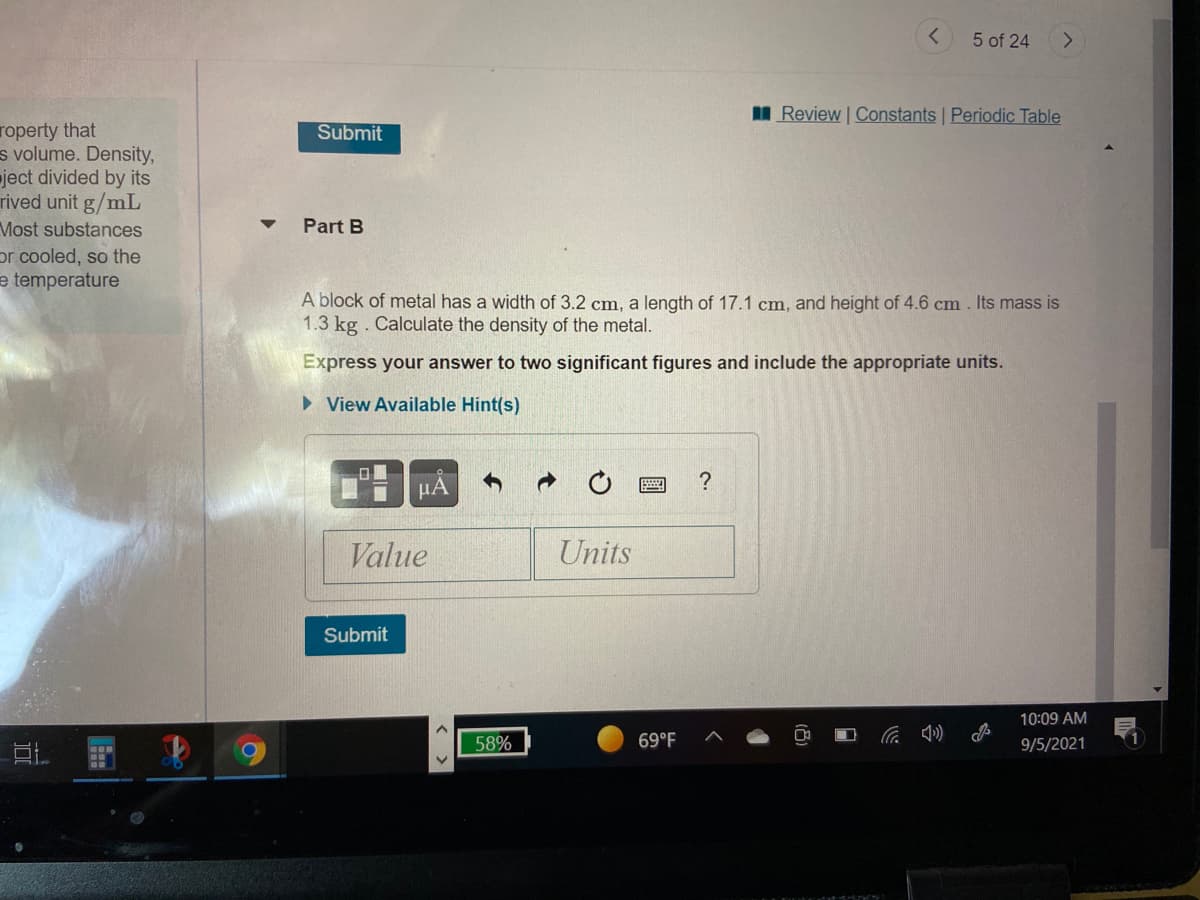
Part B
e temperature
A block of metal has a width of 3.2 cm, a length of 17.1 cm, and height of 4.6 cm . Its mass is
1.3 kg . Calculate the density of the metal.
Express your answer to two significant figures and include the appropriate units.
> View Available Hint(s)
?
HA
Value
Units
Submit
10:09 AM
58%
69°F
9/5/2021
Transcribed Image Text:Q Chen x
O (653 X
20penVellumHMAC=f539db6772ba1c5d7ca97064595a0cbe#10001
O New
+
M Gmail
D YouTube
AMaps
63 Translate
R
Course Home
CHM
国 Readin
<HW Chp 1
+ Density Calculations
5 of 24
Review | Constants Periodic Table
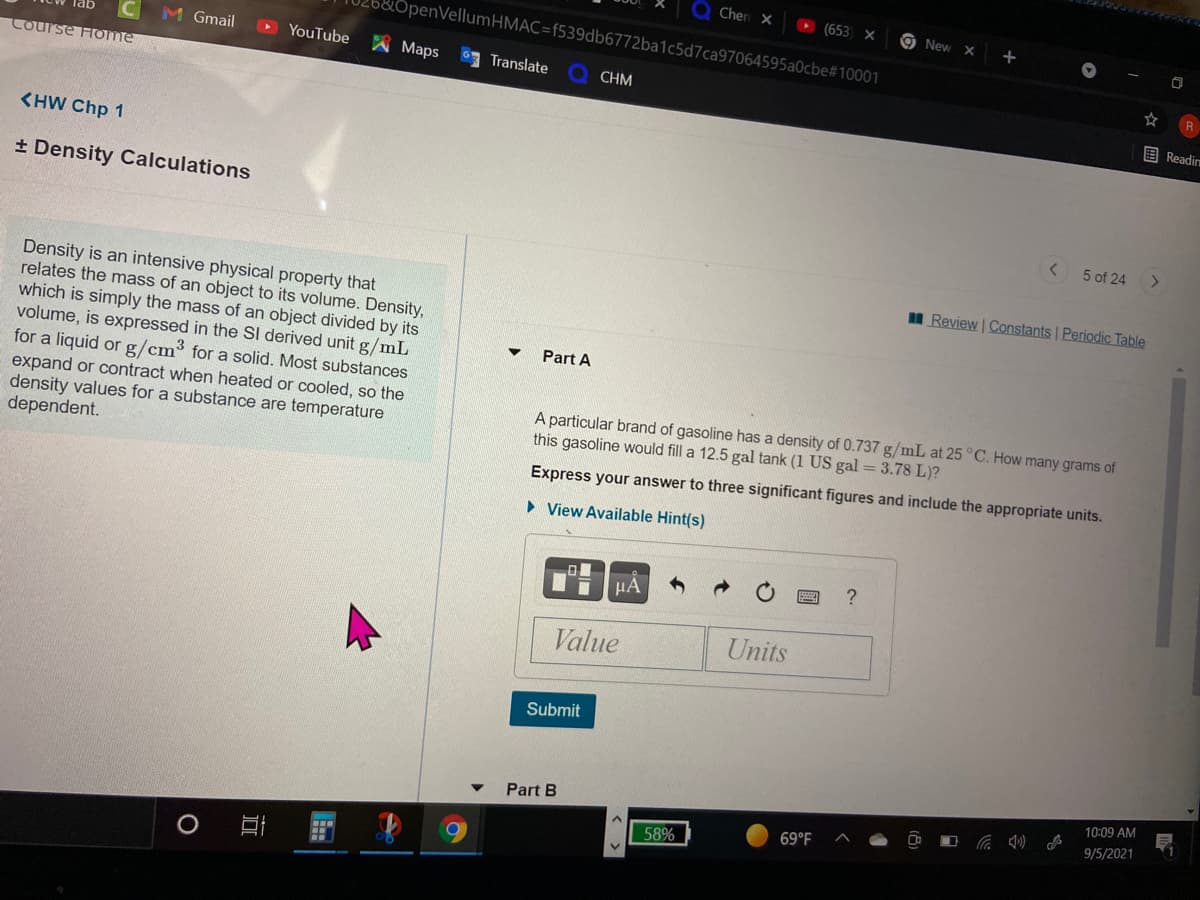
Density is an intensive physical property that
relates the mass of an object to its volume. Density,
which is simply the mass of an object divided by its
volume, is expressed in the SI derived unit g/mL
for a liquid or g/cm3 for a solid. Most substances
expand or contract when heated or cooled, so the
density values for a substance are temperature
dependent.
Part A
A particular brand of gasoline has a density of 0.737 g/mL at 25 °C. How many grams of
this gasoline would fill a 12.5 gal tank (1 US gal = 3.78 L)?
Express your answer to three significant figures and include the appropriate units.
• View Available Hint(s)
µA
Value
Units
Submit
10:09 AM
Part B
9/5/2021
69°F
58%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

