5. Refer to Model 2 for information relevant to this question. A sample of the peptide Lys net charge of zero between what two pH values? What is the pI of Lys-Glu-Ser?
5. Refer to Model 2 for information relevant to this question. A sample of the peptide Lys net charge of zero between what two pH values? What is the pI of Lys-Glu-Ser?
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
100%
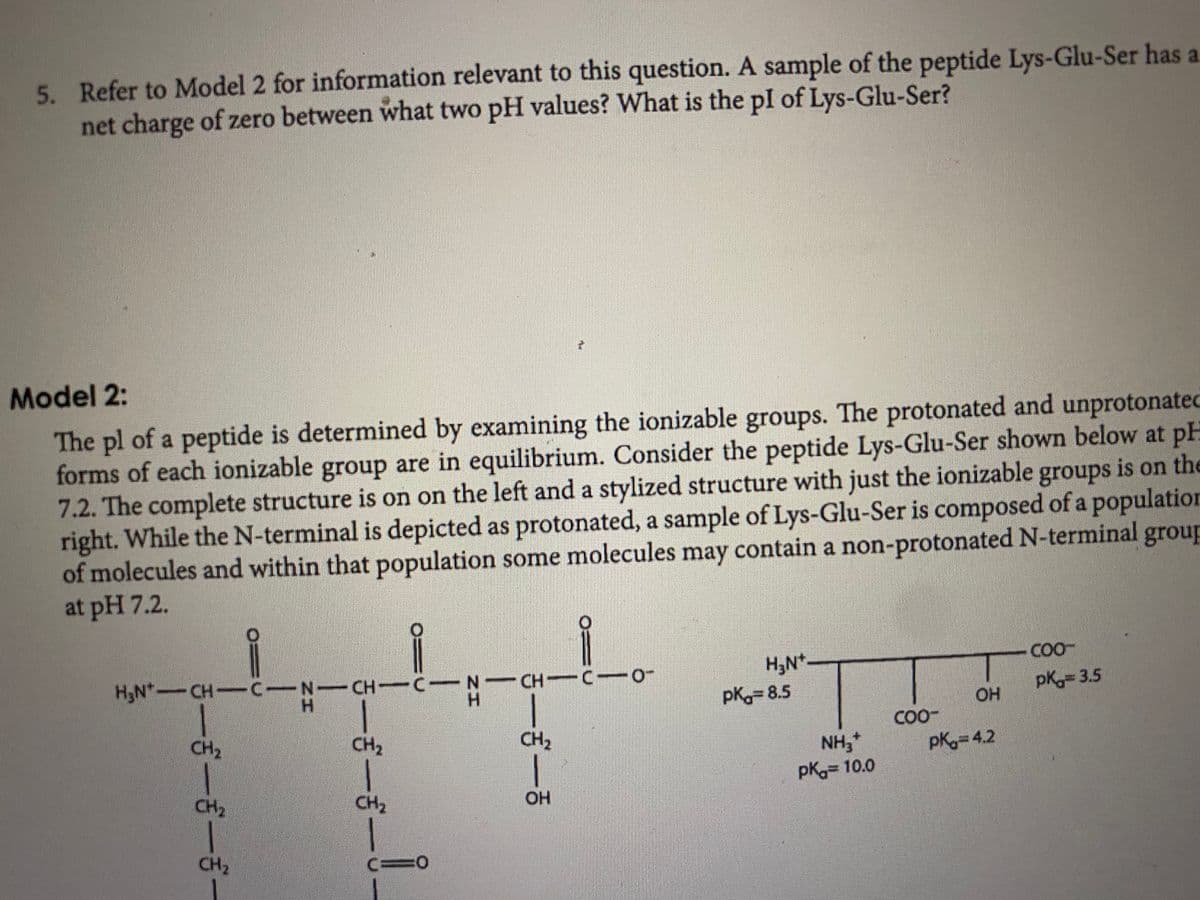
Transcribed Image Text:5. Refer to Model 2 for information relevant to this question. A sample of the peptide Lys-Glu-Ser has a
net charge of zero between what two pH values? What is the pl of Lys-Glu-Ser?
Model 2:
The pl of a peptide is determined by examining the ionizable groups. The protonated and unprotonated
forms of each ionizable group are in equilibrium. Consider the peptide Lys-Glu-Ser shown below at pH
7.2. The complete structure is on on the left and a stylized structure with just the ionizable groups is on the
right. While the N-terminal is depicted as protonated, a sample of Lys-Glu-Ser is composed of a population
of molecules and within that population some molecules contain a non-protonated N-terminal group
at pH 7.2.
may
H3N*
COO-
H3N-CH-CIN-CH C N-CH C-
H.
pK 3.5
OH
H.
pK= 8.5
COO-
CH2
CH2
CH2
NH,
pK 10.0
pK 4.2
CH2
CH2
OH
CH2
c=0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON