created Is BF, being created or destroyed by the chemical reaction? destroyed neither created nor destroyed If BF, is being created or destroyed, what is the rate at which it is being created or destroyed 1100 seconds after the reaction starts? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. If BF, is being created or destroyed, what is the average rate at which it is being created or destroyed during the first 1100 seconds of the reaction? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol.
created Is BF, being created or destroyed by the chemical reaction? destroyed neither created nor destroyed If BF, is being created or destroyed, what is the rate at which it is being created or destroyed 1100 seconds after the reaction starts? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. If BF, is being created or destroyed, what is the average rate at which it is being created or destroyed during the first 1100 seconds of the reaction? Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter14: Chemical Equilibirum
Section: Chapter Questions
Problem 14.122QP: A chemist wants to prepare phosgene, COCl2, by the following reaction: CO(g)+Cl2(g)COCl2(g) He...
Related questions
Question
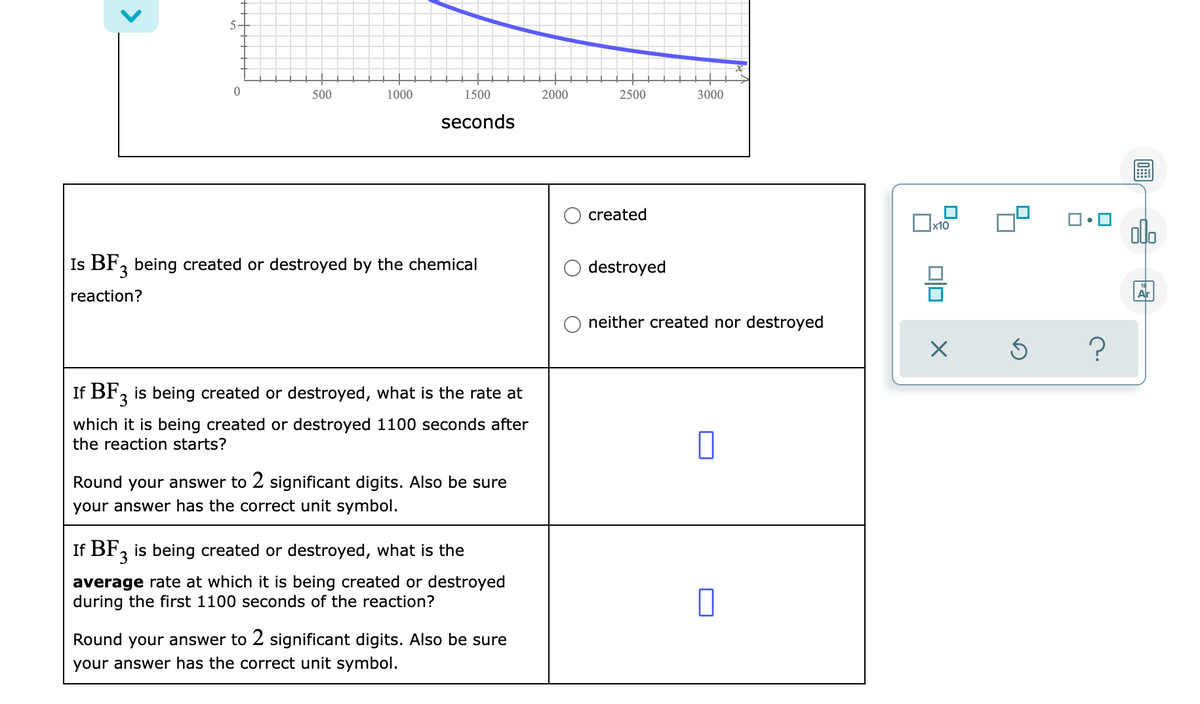
Transcribed Image Text:5-
500
1000
1500
2000
2500
3000
seconds
O created
x10
olo
Is BF, being created or destroyed by the chemical
3
O destroyed
reaction?
Ar
neither created nor destroyed
If BF, is being created or destroyed, what is the rate at
3
which it is being created or destroyed 1100 seconds after
the reaction starts?
Round your answer to 2 significant digits. Also be sure
your answer has the correct unit symbol.
If BF, is being created or destroyed, what is the
average rate at which it is being created or destroyed
during the first 1100 seconds of the reaction?
Round your answer to 2 significant digits. Also be sure
your answer has the correct unit symbol.
>
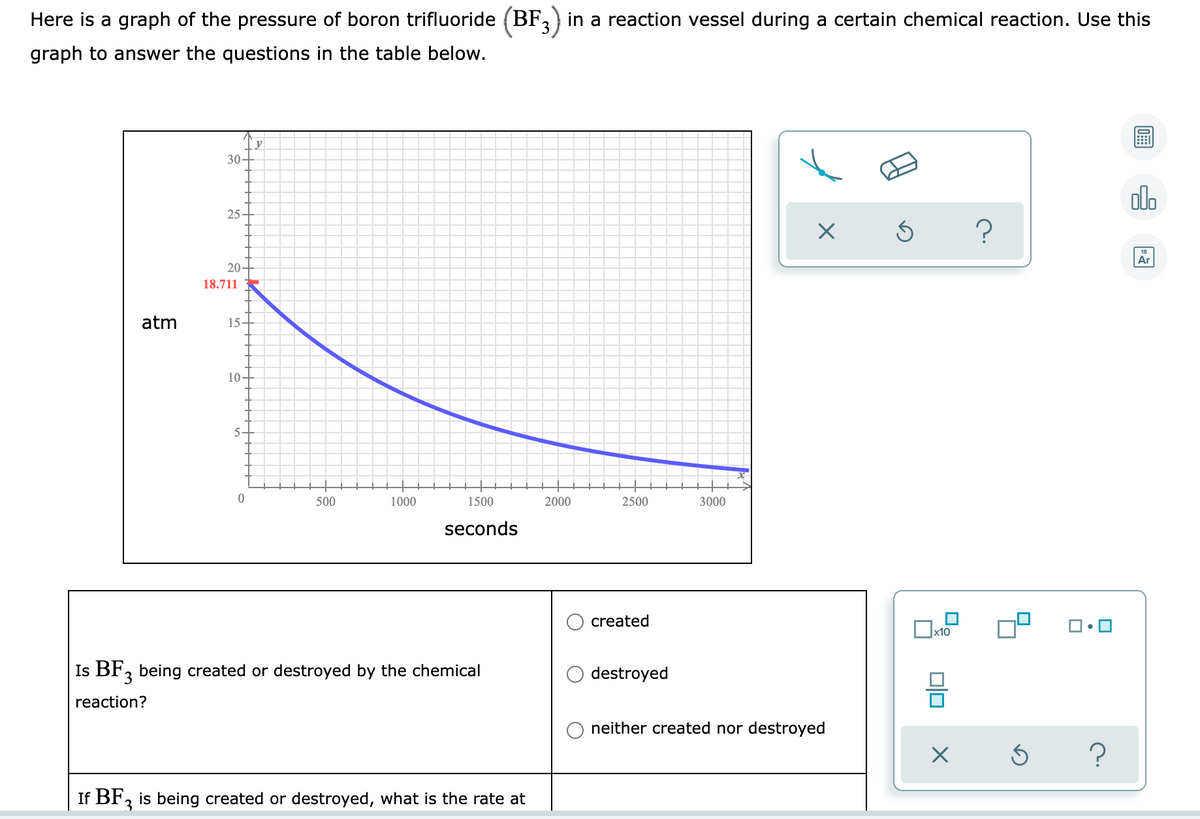
Transcribed Image Text:Here is a graph of the pressure of boron trifluoride (BF, ) in a reaction vessel during a certain chemical reaction. Use this
graph to answer the questions in the table below.
y
30-
olo
25-
?
18
Ar
20-
18.711
atm
15.
10-
5
500
1000
1500
2000
2500
3000
seconds
created
|x10
Is BF, being created or destroyed by the chemical
destroyed
reaction?
neither created nor destroyed
?
If BF, is being created or destroyed, what is the rate at
Expert Solution
Step 1
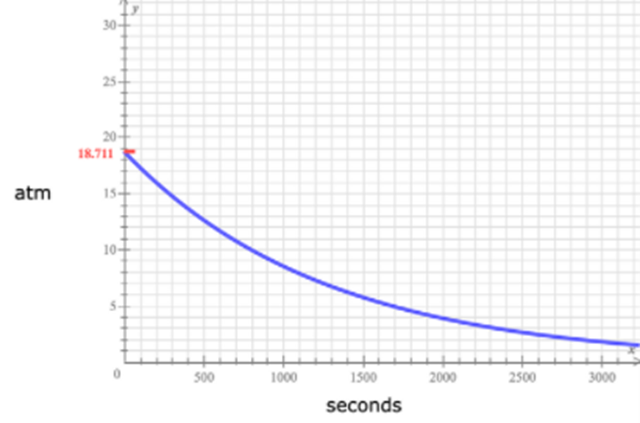
The pressure v/s time curve of BF3 is given as,
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning