***63 Figure 19-27 shows a cycle un- dergone by 1.00 mol of an ideal monatomic gas. The temperatures are T = 300 K, T2 = 600 K, and T3= 455 K. For 1- 2, what are (a) heat Q, (b) the change in internal energy AEnt- and (c) the work done W? For 2 3, what are (d) Q. (e) AEints and (f) W? For 3 → 1, what are (g) Q, (h) AEints and (i) W? For the full cycle, what are (j) Q. (k) AEnt, and (1) W? The initial pressure at point 1 is 1.00 atm (= 1.013 x 10 Pa). What are the (m) volume and (n) pressure at point 2 and the (o) volume and (p) pressure at point 3? Adiabatic Volume Figure 19-27 Problem 63. Pressure
***63 Figure 19-27 shows a cycle un- dergone by 1.00 mol of an ideal monatomic gas. The temperatures are T = 300 K, T2 = 600 K, and T3= 455 K. For 1- 2, what are (a) heat Q, (b) the change in internal energy AEnt- and (c) the work done W? For 2 3, what are (d) Q. (e) AEints and (f) W? For 3 → 1, what are (g) Q, (h) AEints and (i) W? For the full cycle, what are (j) Q. (k) AEnt, and (1) W? The initial pressure at point 1 is 1.00 atm (= 1.013 x 10 Pa). What are the (m) volume and (n) pressure at point 2 and the (o) volume and (p) pressure at point 3? Adiabatic Volume Figure 19-27 Problem 63. Pressure
Physics for Scientists and Engineers
10th Edition
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter21: Heat Engines, Entropy, And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 47CP: The compression ratio of an Otto cycle as shown in Figure 21.12 is VA/VB = 8.00. At the beginning A...
Related questions
Question
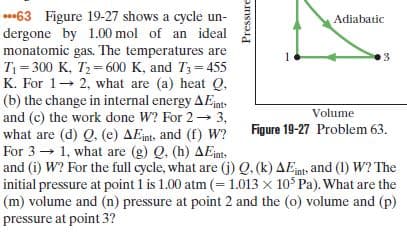
Transcribed Image Text:***63 Figure 19-27 shows a cycle un-
dergone by 1.00 mol of an ideal
monatomic gas. The temperatures are
T = 300 K, T2 = 600 K, and T3= 455
K. For 1- 2, what are (a) heat Q,
(b) the change in internal energy AEnt-
and (c) the work done W? For 2 3,
what are (d) Q. (e) AEints and (f) W?
For 3 → 1, what are (g) Q, (h) AEints
and (i) W? For the full cycle, what are (j) Q. (k) AEnt, and (1) W? The
initial pressure at point 1 is 1.00 atm (= 1.013 x 10 Pa). What are the
(m) volume and (n) pressure at point 2 and the (o) volume and (p)
pressure at point 3?
Adiabatic
Volume
Figure 19-27 Problem 63.
Pressure
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers: Foundations…
Physics
ISBN:
9781133939146
Author:
Katz, Debora M.
Publisher:
Cengage Learning
