7. Soluble oxide like Na2O react with water to form hydroxide For each of these statements, write the: A. Balanced chemical reaction 3. Type of chemical reaction that occurred 8. CO2 react with water to form an acid 9. SO2 react with water to form an acid 10. N2Os react with water to form an acid 11. The combustion of ethanol (C2H5OH) forms carbon dioxide and water vapor. 12. Aqueous solutions of lead(II) nitrate and sodium phosphate are mixed, resulting in the precipitate formation of lead(II) phosphate with aqueous sodium nitrate as the other product. 13. Solid zinc reacts with aqueous HCl to form aqueous zinc chloride and hydrogen gas. 14. Aqueous strontium hydroxide reacts with aqueous hydrobromic acid to produce water and aqueous strontium bromide. 15. Glucose (C6H|206) reacts with oxygen gas to produce gaseous carbon dioxide and water vapor.
7. Soluble oxide like Na2O react with water to form hydroxide For each of these statements, write the: A. Balanced chemical reaction 3. Type of chemical reaction that occurred 8. CO2 react with water to form an acid 9. SO2 react with water to form an acid 10. N2Os react with water to form an acid 11. The combustion of ethanol (C2H5OH) forms carbon dioxide and water vapor. 12. Aqueous solutions of lead(II) nitrate and sodium phosphate are mixed, resulting in the precipitate formation of lead(II) phosphate with aqueous sodium nitrate as the other product. 13. Solid zinc reacts with aqueous HCl to form aqueous zinc chloride and hydrogen gas. 14. Aqueous strontium hydroxide reacts with aqueous hydrobromic acid to produce water and aqueous strontium bromide. 15. Glucose (C6H|206) reacts with oxygen gas to produce gaseous carbon dioxide and water vapor.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter20: Transition Metals And Coordination Chemistry
Section: Chapter Questions
Problem 70E
Related questions
Question
kindly answer 14 and 15. thank you.
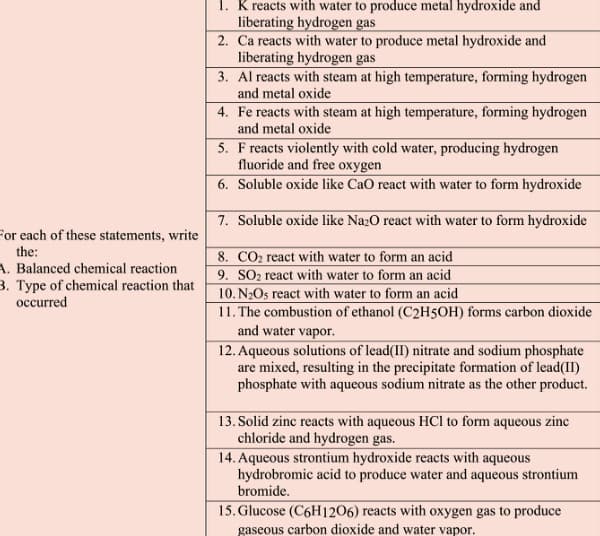
Transcribed Image Text:1. K reacts with water to produce metal hydroxide and
liberating hydrogen gas
2. Ca reacts with water to produce metal hydroxide and
liberating hydrogen gas
3. Al reacts with steam at high temperature, forming hydrogen
and metal oxide
4. Fe reacts with steam at high temperature, forming hydrogen
and metal oxide
5. F reacts violently with cold water, producing hydrogen
fluoride and free oxygen
6. Soluble oxide like CaO react with water to form hydroxide
7. Soluble oxide like Na¿O react with water to form hydroxide
For each of these statements, write
the:
8. CO2 react with water to form an acid
9. SO2 react with water to form an acid
10. N2Os react with water to form an acid
11. The combustion of ethanol (C2H5OH) forms carbon dioxide
and water vapor.
12. Aqueous solutions of lead(II) nitrate and sodium phosphate
are mixed, resulting in the precipitate formation of lead(II)
phosphate with aqueous sodium nitrate as the other product.
A. Balanced chemical reaction
3. Type of chemical reaction that
occurred
13. Solid zinc reacts with aqueous HCl to form aqueous zinc
chloride and hydrogen gas.
14. Aqueous strontium hydroxide reacts with aqueous
hydrobromic acid to produce water and aqueous strontium
bromide.
15. Glucose (C6H1206) reacts with oxygen gas to produce
gaseous carbon dioxide and water vapor.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning