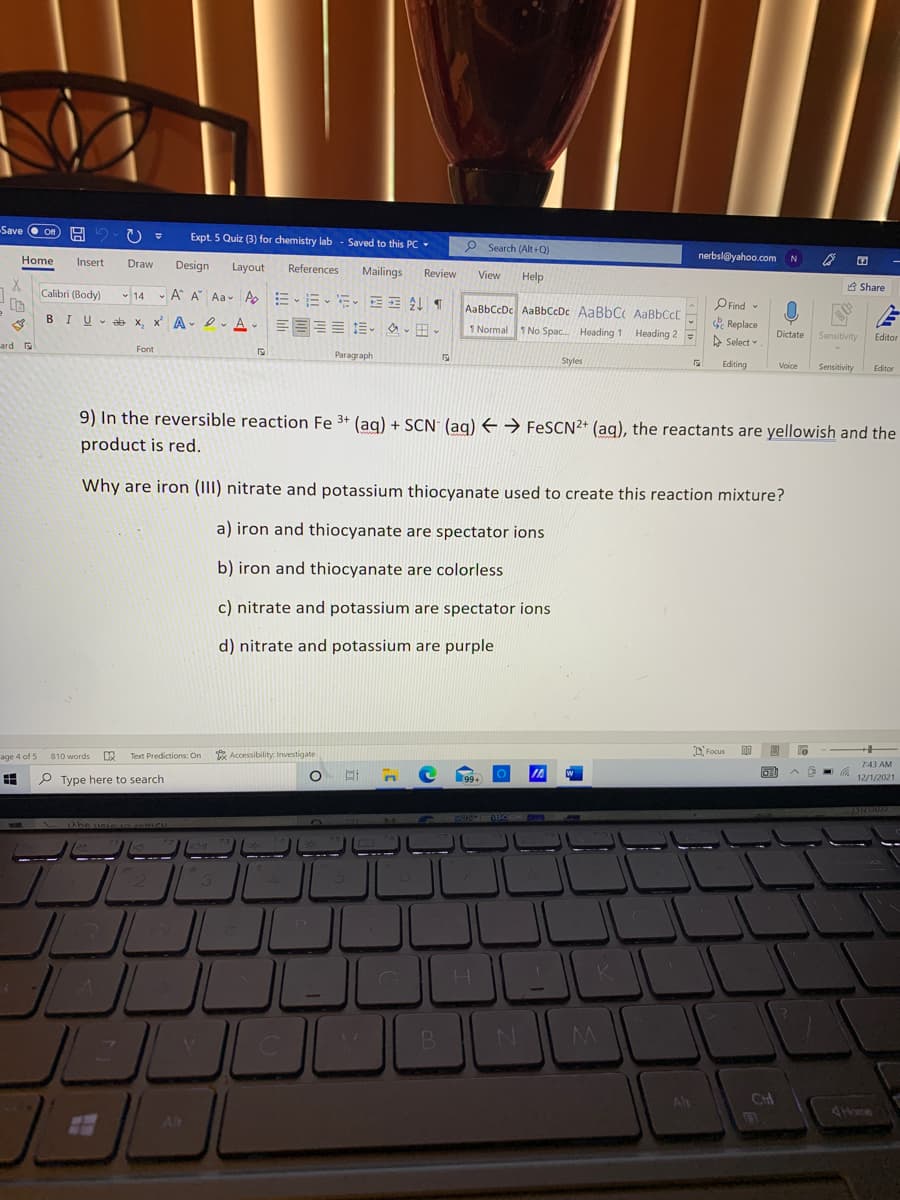
9) In the reversible reaction Fe 3+ (ag) + SCN: (aq) E → FeSCN2* (aq), the reactants are yellowish and the product is red. Why are iron (1II) nitrate and potassium thiocyanate used to create this reaction mixture? a) iron and thiocyanate are spectator ions b) iron and thiocyanate are colorless c) nitrate and potassium are spectator ions d) nitrate and potassium are purple
9) In the reversible reaction Fe 3+ (ag) + SCN: (aq) E → FeSCN2* (aq), the reactants are yellowish and the product is red. Why are iron (1II) nitrate and potassium thiocyanate used to create this reaction mixture? a) iron and thiocyanate are spectator ions b) iron and thiocyanate are colorless c) nitrate and potassium are spectator ions d) nitrate and potassium are purple
Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 12P
Related questions
Question
Transcribed Image Text:Save O Of
Expt. 5 Quiz (3) for chemistry lab - Saved to this PC -
P Search (Alt +Q)
Home
Insert
nerbsl@yahoo.com N
Draw
Design
Layout
References
Mailings
Review
View
Help
8 Share
Calibri (Body)
- 14 A A Aa- ApE-E-E, E E I
AaBbCcDc AaBbCcDc AaBbC AaBbCcC
PFind -
BIU- ae x, x A- D. A
Replace
1 Normal
1 No Spac. Heading 1
Heading
Dictate
Sensitivity
Editor
ard s
A Select
Font
Paragraph
Styles
Editing
Voice
Sensitivity
Editor
9) In the reversible reaction Fe 3+ (ag) + SCN (ag) E → FESCN2+ (aq), the reactants are yellowish and the
product is red.
Why are iron (III) nitrate and potassium thiocyanate used to create this reaction mixture?
a) iron and thiocyanate are spectator ions
b) iron and thiocyanate are colorless
c) nitrate and potassium are spectator ions
d) nitrate and potassium are purple
* Accessibility: Investigate
O Focus
age 4 of 5
810 words
Text Predictions: On
7:43 AM
P Type here to search
12/1/2021
G.
K
Alt
Cid
Alt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning