HC2H302 Co2 NaHCOз NaC2H302 + H20 + acetic acid + sodium bicarbonate sodium acetate + water + carbon dioxide melp his brother make a science fair volcano. experiment, Samuel obtains samples of solid acetic acid powder and solid sodium bicarbonate powder from his chemistry teacher. He mixes, the two powders together, but there is no reaction. ring the state of matter of the particles, give a possible explanation for why there is no reaction.
HC2H302 Co2 NaHCOз NaC2H302 + H20 + acetic acid + sodium bicarbonate sodium acetate + water + carbon dioxide melp his brother make a science fair volcano. experiment, Samuel obtains samples of solid acetic acid powder and solid sodium bicarbonate powder from his chemistry teacher. He mixes, the two powders together, but there is no reaction. ring the state of matter of the particles, give a possible explanation for why there is no reaction.
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.15QAP
Related questions
Question
Explain why that combination of temperature and vinegar concentration would achieve the results Samuel is looking for. In your explanation be sure to discuss the effect of each reaction condition on the individual reacting particles for a slow oozing reaction
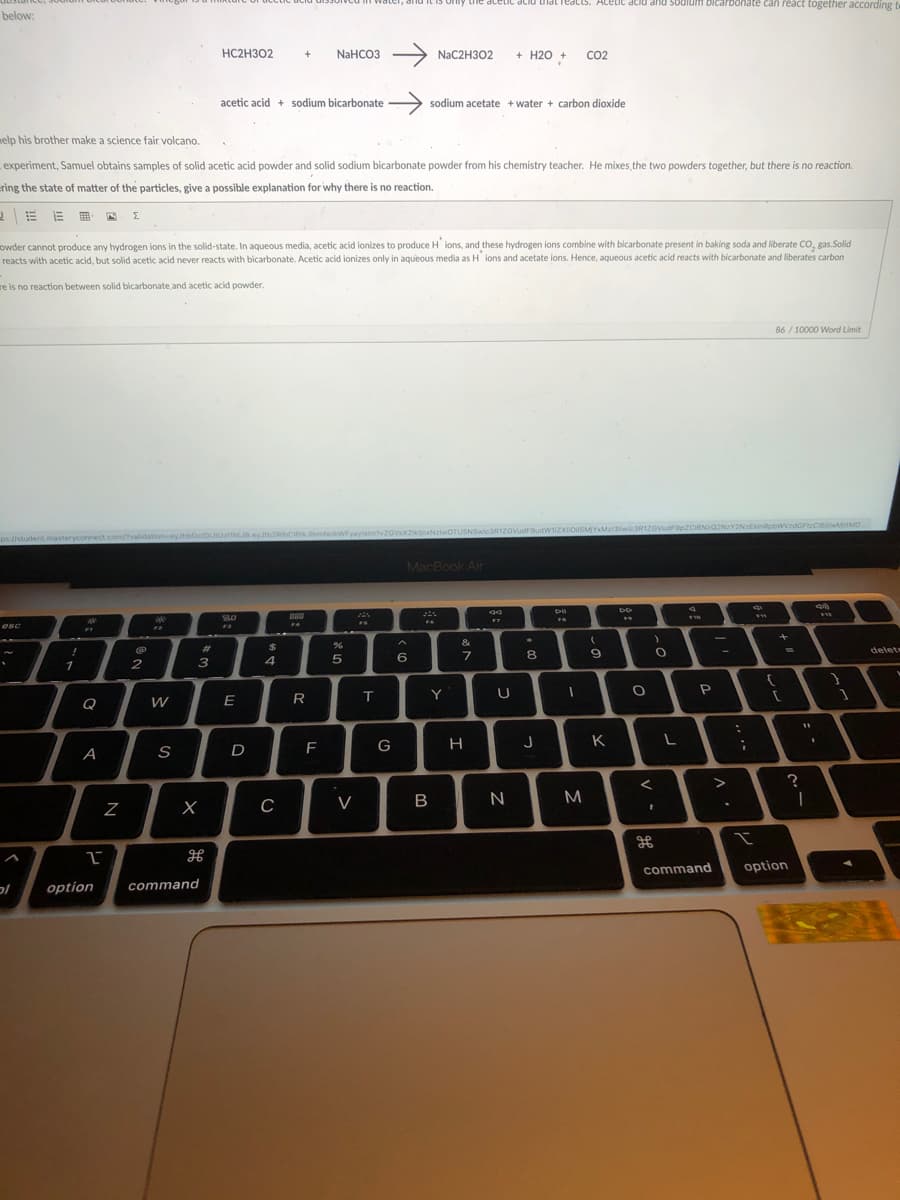
Transcribed Image Text:Lhe acetiL aciu tldtTeacts. Acetic acid and sodium bicarbonate can react together aCcording t
below:
HC2H302
NaHCO3 >
NAC2H302
+
+ H20 +
CO2
acetic acid + sodium bicarbonate
sodium acetate + water + carbon dioxide
-
elp his brother make a science fair volcano.
experiment, Samuel obtains samples of solid acetic acid powder and solid sodium bicarbonate powder from his chemistry teacher. He mixes the two powders together, but there is no reaction.
ring the state of matter of the particles, give a possible explanation for why there is no reaction.
2|m市
Σ
owder cannot produce any hydrogen ions in the solid-state. In aqueous media, acetic acid ionizes to produce H ions, and these hydrogen ions combine with bicarbonate present in baking soda and liberate CO, gas.Solid
reacts with acetic acid, but solid acetic acid never reacts with bicarbonate. Acetic acid ionizes only in aqueous media as H ions and acetate ions. Hence, aqueous acetic acid reacts with bicarbonate and liberates carbon
re is no reaction between solid bicarbonate and acetic acid powder.
86 / 10000 Word Limit
RobwVzdGFtoCI6wO
MiMD
BR1ZG
ps://student.masteryconnect.com/?validatio
HUZINUS.ey.tb2RIbCIGikJlbmNobWFyayisimtvZGVsX2joxNzlwOTUSNSwic3R1ZGVudF9udW1iZxiIoISMYMzi3
MacBook Air
DO
FS
esc
&
%23
%24
delete
8
2
P
Y
Q
G
H
J
K
A
D
M
C
V
H
command
option
option
command
B
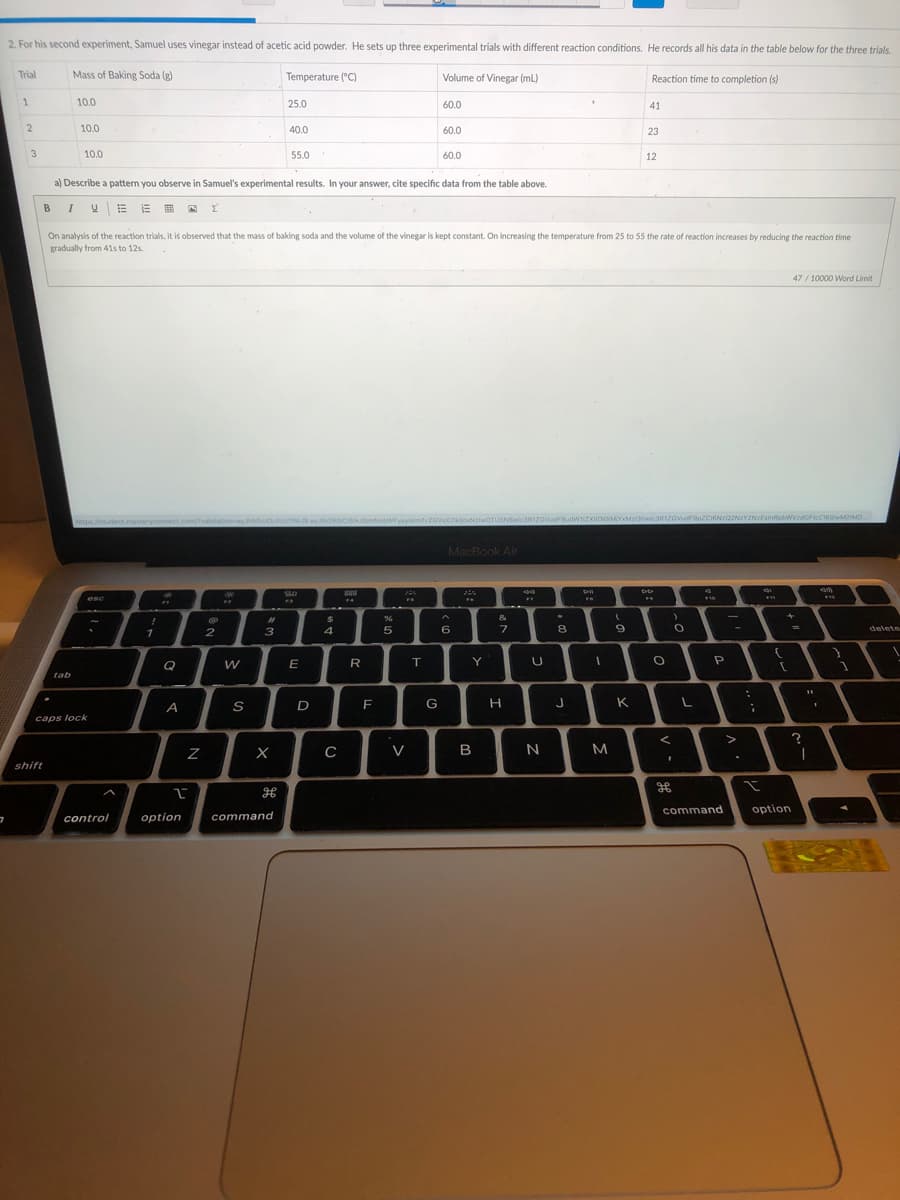
Transcribed Image Text:2. For his second experiment, Samuel uses vinegar instead of acetic acid powder. He sets up three experimental trials with different reaction conditions. He records all his data in the table below for the three trials.
Trial
Mass of Baking Soda (g)
Temperature ("C)
Volume of Vinegar (mL)
Reaction time to completion (s)
10.0
25.0
60.0
41
10.0
40.0
60.0
23
3
10.0
55.0
60.0
12
a) Describe a pattern you observe in Samuel's experimental results. In your answer, cite specific data from the table above.
BIUEE I A
Σ
On analysis of the reaction trials, it is observed that the mass of baking soda and the volume of the vinegar is kept constant. On increasing the temperature from 25 to 55 the rate of reaction increases by reducing the reaction time
gradually from 41s to 12s.
47 / 10000 Word Limit
obWFyayisimlvzovsX2ikjoxNzlwOTUSNS
:3R1ZOVudF9udWizXio5MYXMzi3iwic3R1ZOVudF9pZCION2Q21
zdGFtcCi6UwMMD
ttps///student.mast
MacBook Air
esc
&
%23
2.
3
6
8
delete
Q
E
R
T.
Y
tab
D
F
G
K.
caps lock
V
B
shift
command
option
control
option
command
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning