9. Spectral lines are shown in the image below. But what are they? Explain how spectral lines are formed based upon what you learned in the Flame Test Lab. Spectrometer: photons emitted / nm 500 600 700 780 IR 7500 92 UV 380
9. Spectral lines are shown in the image below. But what are they? Explain how spectral lines are formed based upon what you learned in the Flame Test Lab. Spectrometer: photons emitted / nm 500 600 700 780 IR 7500 92 UV 380
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 11ALQ: r Questions 11—13, you will need to consider ionizations beyond the first ionization energy. For...
Related questions
Question
Answer question 9 and please make it short and let your handwriting clear
Transcribed Image Text:at Tools Add-ons Help Accessibility
Last edit was seconds ago
B IUA
GD DO
1三
mal text
Arial
10
+
4
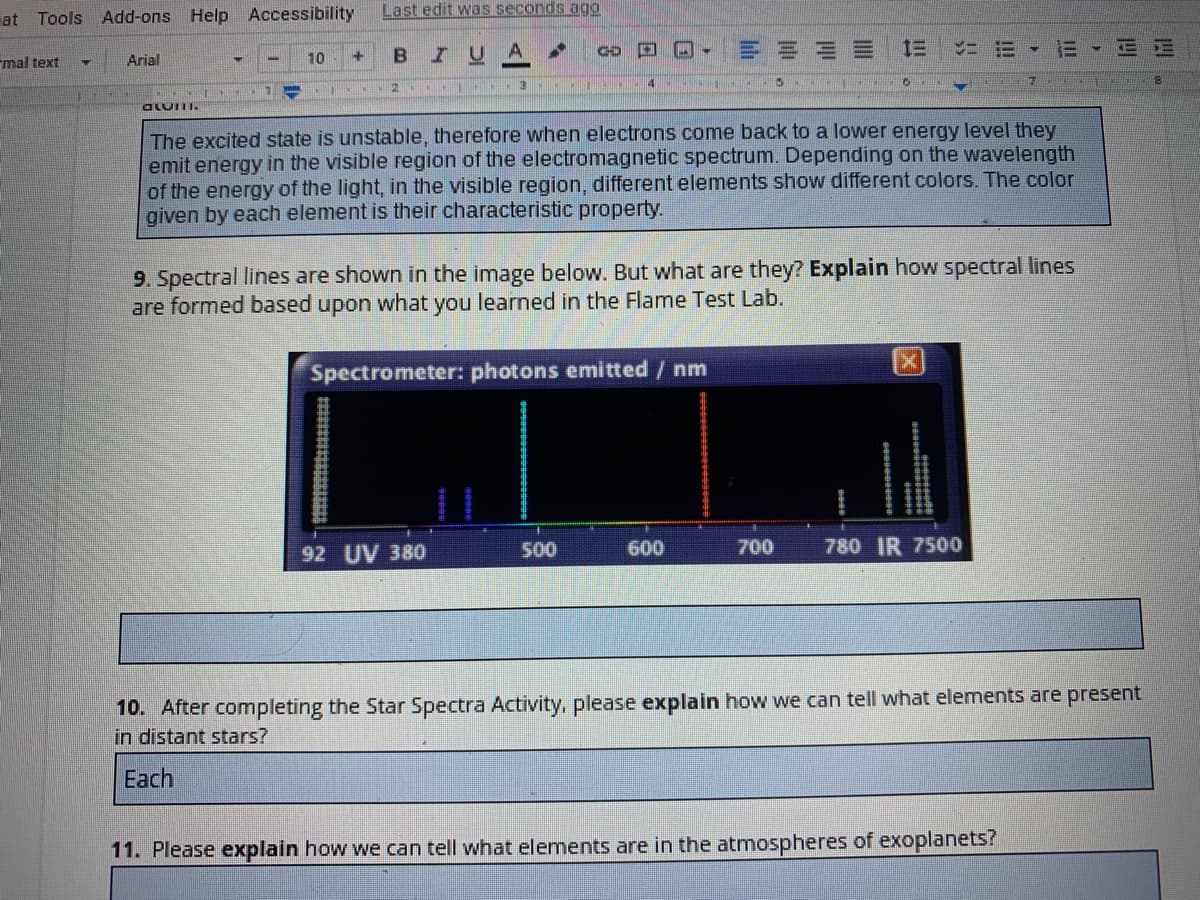
The excited state is unstable, therefore when electrons come back to a lower energy level they
emit energy in the visible region of the electromagnetic spectrum. Depending on the wavelength
of the energy of the light, in the visible region, different elements show different colors. The color
given by each element is their characteristic property.
9. Spectral lines are shown in the image below. But what are they? Explain how spectral lines
are formed based upon what you learned in the Flame Test Lab.
Spectrometer: photons emitted/ nm
92 UV 380
500
600
700
780 IR 7500
10. After completing the Star Spectra Activity, please explain how we can tell what elements are present
in distant stars?
Each
11. Please explain how we can tell what elements are in the atmospheres of exoplanets?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
