A 208 g of C6H6 (78 g/mol.) burns in air according to the equation: CSH6 + O2 C02 +H2O What mass of CO2 (44 g/mol.) is produced?
A 208 g of C6H6 (78 g/mol.) burns in air according to the equation: CSH6 + O2 C02 +H2O What mass of CO2 (44 g/mol.) is produced?
Solid Waste Engineering
3rd Edition
ISBN:9781305635203
Author:Worrell, William A.
Publisher:Worrell, William A.
Chapter7: Thermal Processes
Section: Chapter Questions
Problem 7.2P: The ideal equation for the combustion of cellulose is C6H10O5 + 6O26O2 + 5H2O. What is a similar...
Related questions
Question
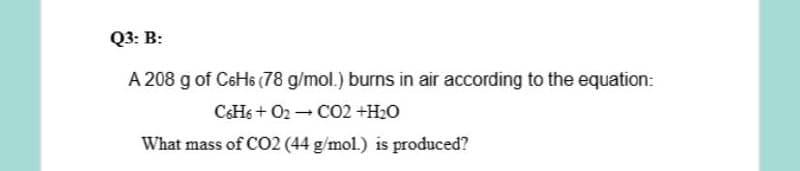
Transcribed Image Text:Q3: B:
A 208 g of C6H6 (78 g/mol.) burns in air according to the equation:
CHs + O2 - CO2 +H2O
What mass of CO2 (44 g/mol.) is produced?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, civil-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Solid Waste Engineering
Civil Engineering
ISBN:
9781305635203
Author:
Worrell, William A.
Publisher:
Cengage Learning,


Solid Waste Engineering
Civil Engineering
ISBN:
9781305635203
Author:
Worrell, William A.
Publisher:
Cengage Learning,
