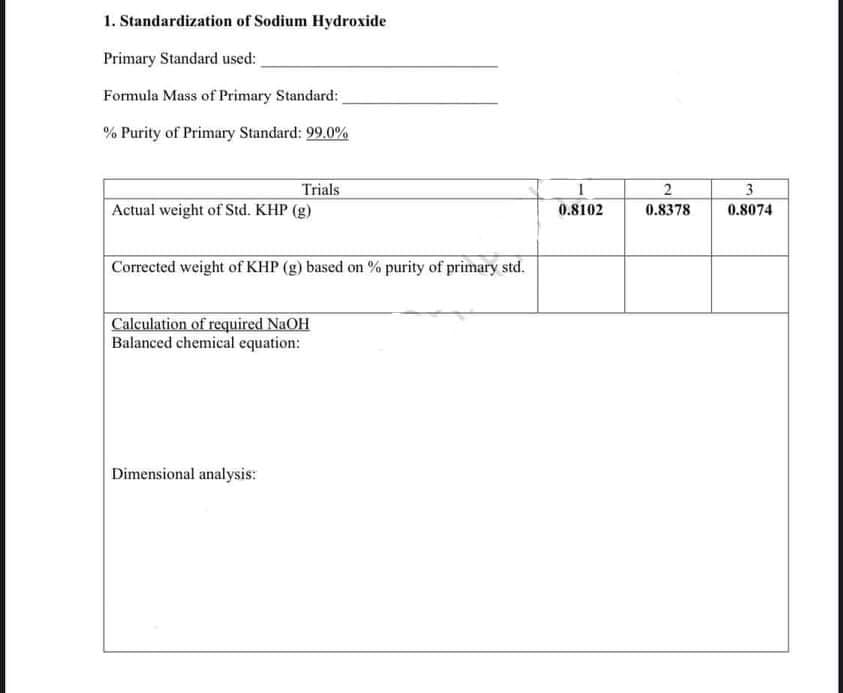
1. Standardization of Sodium Hydroxide Primary Standard used: Formula Mass of Primary Standard: % Purity of Primary Standard: 99.0% Trials Actual weight of Std. KHP (g) 2 3 0.8102 0.8378 0.8074 Corrected weight of KHP (g) based on % purity of primary std. Calculation of required NaOH Balanced chemical equation: Dimensional analysis:
Q: The standard addition method is used to analyze a sample of a river water for mercury. Solution A is…
A: A question based on tools in analytical chemistry that is to be accomplished.
Q: The standard addition method is used to analyze a sample of a river water for mercury. Solution A is…
A: Given, Percent transmittance value of solution A = 56 % Percent transmittance value of solution B =…
Q: Which sentence is false about gravimetric analysis? a. It is used for inorganic b. It is used to…
A: The statement which is false about gravimetric analysis is, Relative precision 3% to 4%
Q: The standard addition method is used to analyze a sample of a river water for mercury. Solution A is…
A: A question based on tools in analytical chemistry that is to be accomplished.
Q: Why is it important that the chemical used as the primary standard is non- hygroscopic and pure? Why…
A: Given statements, It is important that the chemical used as the primary standard is non-…
Q: B. DAY 2. Percent Acetylsalicylic Acid in the Aspirin Sample Calculation for the mass of aspirin for…
A: Given: The molarity of the NaOH solution is 0.1 mol/L. The mass of the aspirin sample taken in trial…
Q: 100 ml boiled cooled and filtered water sample takes 9.6 ml of M/50 EDTA in titration. The…
A: The hardness degree can be calculated in term of CaCO3 equivalent asDegree of Hardness=Wt. of…
Q: You are required to prepare working standard solutions of 1.00 × 10−5, 2.00 × 10−5, 5.00 × 10−5, and…
A: Given M₁ = 0.100M stock solution V₁ = volume of Stock solution M₂ = Working standard V₂ = Volume of…
Q: Scientist 1 analyzed the compounds first then gave it to scientist 2 for the next test. Scientist 2…
A: A question based on concentration terms that is to be accomplished.
Q: For each of the following situations, choose the best measuring apparatus that can be used.…
A: The given question discusses about the accuracy of several balance and glasswares in order to make…
Q: The concentration of chloride in seawater can be determined by a flow injection analysis. The…
A: To find concentration of Cl- in the sample at an absorbance of 0.443.
Q: A 100.0-ml sample of boiler feed water, analyzed for hardness, required 36.8 ml of 0.0386 M EDTA to…
A:
Q: An instrumental method was validated by running an analysis on a standard solution with a known…
A: Accuracy: It is generally expressed by the percentage of error. Relative accuracy is the difference…
Q: Sources of Error Determine the relationship between the observed/apparent value (EX) VERSUS that of…
A: Given: Not all the solid was transferred during the filtration of the precipitate.
Q: Color BEFORE adding Color AFTER adding Intensity of color AFTER adding FeCl3 Test Tube # FeCl3 FeCl3…
A: Using a melting point apparatus, Determine the melting point of obtained crude aspirin and…
Q: A 100.0-ml sample of boiler feed water, analyzed for hardness, required 23.8 ml of 0.0146 M EDTA to…
A:
Q: In a gravimetric chloride analysis, it was found that 0.82g AgCl and 0.80g AgCl was obtained from an…
A: Moles = mass / molecular mass molecular mass of AgCl = 108 + 35.5 = 143.5 => moles of AgCl in…
Q: question 8 - Calculate the percent error for the separation of your mixture- equation 3 How can I…
A: It is given that the observations in the given table are recorded and percent error and the mean…
Q: The standard addition method is used to analyze a sample of a river water for mercury. Solution A is…
A: Given, Percent transmittance of solution A = 56 % Percent transmittance of solution B = 33 % Percent…
Q: The primary standard substance must be less equivalent weight (or molecular Wt. ) False True
A: Primary standard substance are used to standardized the secondary materials or solutions which is…
Q: mass of dish 1631.5 g mass of dish and mix 1822 g mass of dish and agg. after extraction…
A: We have find out the volume of the solvent.
Q: The standard addition method is used to analyze a sample of a river water for mercury. Solution A is…
A: Cold vapour atomic absorption spectroscopy or CVAAS is one of the primary techniques for mercury…
Q: nL of the unknown were pipetted into each of five 50.0-ml volumetric flasks. Various volumes of a…
A:
Q: The concentration of chloride in seawater can be determined by a flow injection analysis. The…
A: We are given Cl- concentration and absorbance data for calibration standards. We have to find Cl-…
Q: When answering this problem, report the answer with the appropriate number of significant figures.…
A: Given : Calibration curve equation : y = 21655 x + 0.0318 where y = Absorbance x = molar…
Q: Arrange the following steps in performing a general procedure. I. work on the data II. Carry out the…
A: we are required to outline the steps to perform a general procedure
Q: A student weighed approximately 1.5 g of an unknown solid acid and carefully placed the sample in a…
A: Let the dibasic acid be H2Y It will be neutralized by NaOH in the following way:- H2Y + 2NaOH =…
Q: Given the following data Dissolved Oxygen Titration Data Trial 1 Preparation of titrant 0.4455 g…
A:
Q: In this experiment, the mass of pure copper provided is 0.491 gram, and then it was dissolved in…
A: Given Mass of pure Copper is 0.491 g. Volume of original solution as 100 mL. Volume of diluted…
Q: I got a task in analytical chemistry to calibrate, standardize, verify and validate an analytical…
A: It is said that every location in the world is positioned differently in relation to magnetic…
Q: The standard addition method is used to analyze a sample of a river water for mercury. Solution A is…
A: In the above question, mecury Determination have to perform in undiluted sample. Most common method…
Q: Standardization of EDTA Solution Weight of pure CaCO3: 0.2517 g % Purity of CaCO3: 98.0% Total…
A: The experiment data given is, Trial 1 2 3 The volume of standard CaCO3 solution (ml) 25.00…
Q: 5.00 mL of stock solution is diluted to 25.00 mL, producing solution ALPHA. 10.00 mL of solution…
A: Dilution factor refers to the ratio of initial volume of the concentrated solution to the final…
Q: An environmental chemist working for the Environmental Protection Agency (EPA) was directed to…
A:
Q: In a chromatography experiment, a solution containing 0.083 7 M X and 0.066 6 M S gave peak areas of…
A: The equation to calculate the concentration of unknown using internal standard from a chromatogram…
Q: Which error will lead to negative deviation from the theoretical Keq? a. cuvette was filled to the…
A: Which error will lead to negative deviation from the theoretical Keq. Answer : Correct…
Q: According to ISO/IEC 17025: a. non-standard methods are required to be validated b. method…
A: What is ISO/IEC 17025 ?
Q: 13.00 mL pFe2+ the equivalence point, V. pFe2+ = 17.50 mL pFe?+
A: The question is based on the complexometric titration of ferrous ions and EDTA. we have to calculate…
Q: Jake carried out three titrations to determine the concentration of unknown nitric acid (HNO3) using…
A: [NaOH] = 0.1050 M Vol of HNO3 used = 100.0 ml Vol of Na OH used (Va)= 20.56 ml Vol of Na OH used…
Q: Caffeine, benzoate, and aspartame content of mountain dew soda was determined using reverse phase…
A: Given: Concentration of standard Caffeine used = 0.70 mg/mL Concentration of standard Benzoate used…
Q: ▪ A pharmaceutical analyst needs to do quality control of a sodium furosemide solution. The max for…
A: Absorbance = Absorptivity coefficient × Concentration × Path length Path length = 1 cm Absorptivity…
Q: 2. A water sample from a local cave system was tested for total water hardness. An EDTA titer was…
A: The question is based on the concept of complexometric titrations. We have to calculate hardness of…
Q: potassium hydrogen phthalate is a primary standard used to measure the concentration of NaOH…
A: Due to the buoyancy force by air, the mass of an object measured in the air is always appear to be…
Q: Dissolve 1.7 g in a mixture of 2 ml. of nitric acid (-130 g/l) TS and 40 ml of water, and proceed as…
A: The analyst recorded a weight of 1.8652g of sample using an analytical balance.
Q: 2. Determining the Acid Content of Vinegar Trials A В C Volume of Vinegar used (ml) 25.00 diluted to…
A:
Q: Upon this addition of potassium chromate, you observe no formation of precipitate. Which of the…
A: Pb2+, Ag+, and Hg22+ give a white precipitate with HCl. So the formation of white precipitate with…
Q: concentration of the total suspended solid. In the laboratory, 5 mL of the water sample was…
A: Initial weight of glass fiber filter = 0.2431 g = 243.1 mg and Glass fiber filter + suspended solid…
Q: Explain briefly the percent volume and percent retrieval in separation of mixtures.
A:
Q: 3. Calculate the percent purity of compound X using D and E as recrystallization solvent. % purity =…
A: Given values : Option B is correct answer
Q: Data Sheet with Sample Results Note: results that require calculations are purposely left blank and…
A: (7) Mass of NaCl dissolved : Mass of NaCl dissolved = (Mass of 50mL beaker + Stirring bar + NaCl) -…
Step by step
Solved in 2 steps with 3 images

- A student was given an unknown hydrate, which could be one of threepossibilities: magnesium sulfate heptahydrate, sodium dichromate tetrahydrate orbarium chloride dihydrate. Using the following data identify the unknown.Wt. of crucible/lid empty 39.46 gWt. of crucible/lid/hydrate sample 46.84 gWt. of crucible/lid/sample after heating 45.77 gGiven the following data forMass of test tube, beaker and cyclohexane = 100.17 gMass of test tube and beaker = 84.07 gFreezing point of cyclohexane = 6.59 oCMass of weighing paper + naphthalene =1.080 gMass of weighing paper = 0.928 gFreezing point solution = 5.11oCKf = 20.8oC/mDetermine the followinga. mass of cyclohexane in g (2 decimal places); _____b. mass of naphthalene in g (4 decimal places); _____c. freezing point depression (2 decimal places); _____d. molality of solution (3 significant figures); _____e. moles of naphthalene (3 significant figures); _____f. molar mass of naphthalene, experimentally (3 significant figures); _____g. % error if theoretical molar mass of naphthalene is 128.17 g/ mole, USE ABSOLUTE VALUE (3 significant figure); ____A mixture of ethanol (ethyl alcohol) and water contains 40.0% water by mass.(a) Assuming volume additivity of the components, estimate the specific gravity of themixture at 20°C. What volume (in liters) of this mixture is required to provide 150 mol ofethanol?(b) Repeat Part (a) with the additional information that the specific gravity of the mixture at20°C is 0.89045 (making it unnecessary to assume volume additivity). What percentage errorresults from the volume-additivity assumption?
- Sum of coefficients C7H8 + O2 --> CO2 + H2O after balancingCalculate the Constant Weight (in grams) of the Empty Crucibles. Show your solution. Crucible No. 1 2 3 Weighing 1 22.6035 22.0223 24.1535 Weighing 2 22.6017 22.0204 24.1533 Weighing 3 22.5994 22.0199 - Weighing 4 22.5992 - - Constant Weight, g ??? ??? ???1. Calculate the experimental density of a salt solution and the percent error (same as relative error percent) using some or all the data given below. solubility of NaCl salt in water: 0.357 g/mLmass of empty graduated cylinder: 25.19g mass of graduated cylinder + salt solution: 30.47g total volume of salt solution: 4.98 mLtrue density of salt solution: 1.07 g/mL
- Medicare does not cover the cost of this prescription medicine, which currently averages $297.78 per kilogram. The theoretical yield for the pure barium sulfate you were transferring was 500.00kg. You spilled 27.45 kg. What is the % yield of the transfer that you need to report? And also determine the consumer price of the spilled barium sulfate.Using the percent purity calculations, determine the percent yield of synthesis of aspirin. Part I Synthesis of Aspirin Mass of salicylic acid used (g) 2.029g Volume of acetic anhydride used (mL) 5ml Mass of acetic anhydride used (vol. × 1.08 g/mL) 5.4g Mass of aspirin synthesized (g) 3.256g Part II Melting Temperature Data Melting temperature (°C) 133°C Part III Salicylic Acid Standard Stock Solution Initial mass of salicylic acid (g) 0.210g Moles of salicylic acid (mol) 0.0147 mol Initial molarity of salicylic acid (M) 0.724 M Part III Beer’s Law Data for Salicylic Acid Standard Solutions Trial Concentration (M) Absorbance Water (mL) 1 10 0.301 0 2 7.5 0.219 2.5 3 5.0 0.163 5.0 4 2.5 0.074 7.5 Best-fit line equation for the salicylic acid standards Test of the Purity of the Synthesized Aspirin Initial mass of aliquot of product (g)…125.1 mg of streptomycin sulphate are dissolved in 10 ml of water. A GC headspace analysis is carried out in order to determine the methanol content of the drug. A peak for methanol is produced which has 73.2% of the area of a peak for a methanol standard containing 0.532 mg/100 ml of methanol in water analysed under exactly the same conditions, What is the methanol content of the streptomycin sulphate in ppm and %w/w? Answer: 311.3 ppm, 0.3113 % w/w.how??
- An orange juice processing plant now produces essential oil from orange peels. one It is known that 250 kg of peel comes out of 1 ton of oranges and 2.5 g of essential oil comes out of 1 kg of peel. In a laboratory study, 250 g of bark was treated with hexane solvent and 0.548 g of essential oil was obtained in the sample cup of the rotary evaporator. Accordingly, the rotary Calculate the separation efficiency obtained in the evaporator?If 35,000 kg of whole milk containing 4% fat is to be separated in a 6-hour period into skim milk with 0.45% fat and cream with 45% fat, what are the mass flow rates of the two output streams from a continuous centrifuge which accomplishes this separation? (Ans; Cream=464.8335kg/h, Skim milk= 5368.4998kg/h)Stock iron(II) solution (200Ug mL-1 Fe) ferrous ammonium sulfate hexahydrate mass= 0.1437g, transfer it to a 100 ml beaker. add 15 ml approx of water and 15m1 'approx of dilute sulphuric acid (2M H2SO.). then transfer FeII to 100 ml flask makeup to the mark with water. calculate the moles of ferrous ammonium sulfate hexahydrate solution in unit ug/mL.



