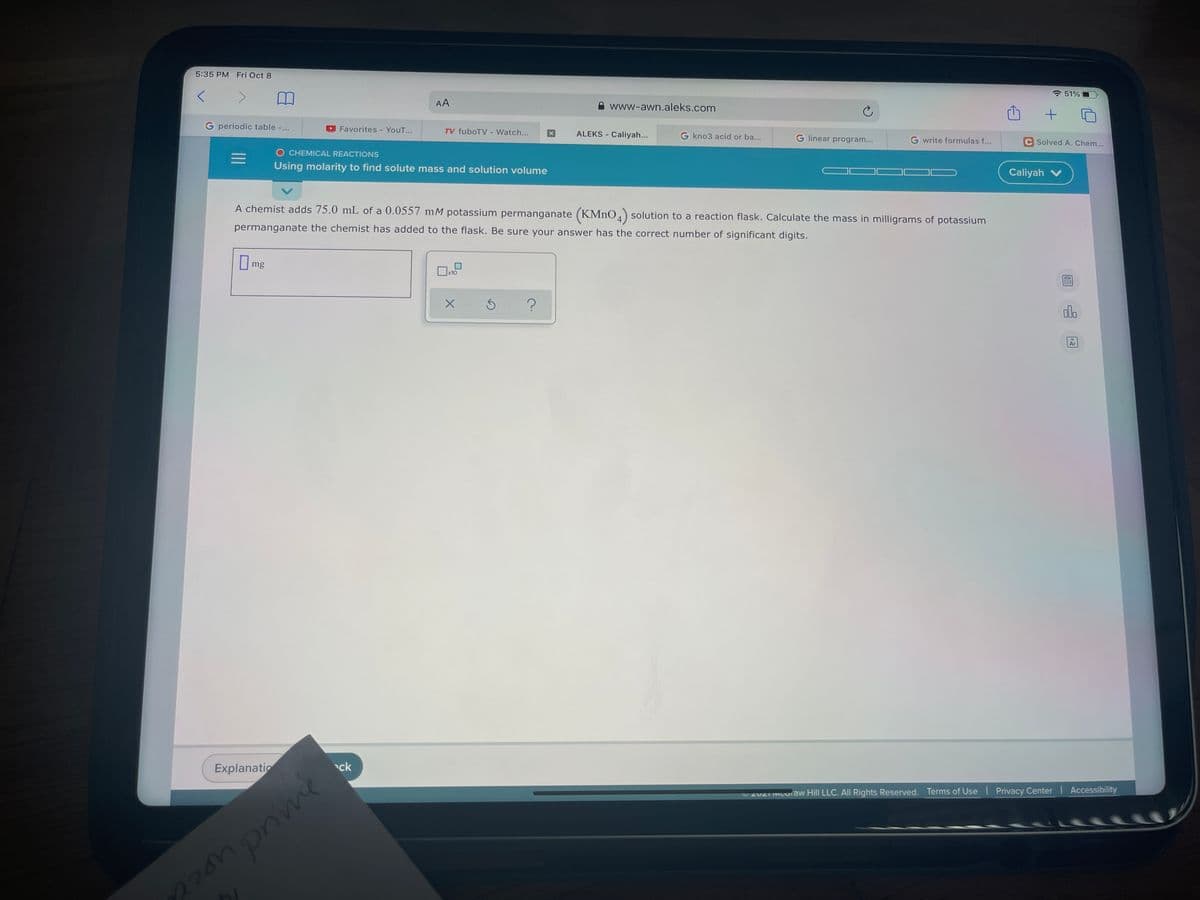
A chemist adds 75.0 mL of a 0.0557 mM potassium permanganate (KMNO, solution to a reaction flask. Calculate the mass in milligrams of potassium permanganate the chemist has added to the flask. Be sure your answer has the correct number of significant digits. me
A chemist adds 75.0 mL of a 0.0557 mM potassium permanganate (KMNO, solution to a reaction flask. Calculate the mass in milligrams of potassium permanganate the chemist has added to the flask. Be sure your answer has the correct number of significant digits. me
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 93QAP
Related questions
Question
Transcribed Image Text:5:35 PM Fri Oct 8
令51%
AA
www-awn.aleks.com
G periodic table -...
Favorites - YouT...
TV fuboTV- Watch...
ALEKS - Caliyah...
G kno3 acid or ba...
G linear program...
G write formulas f...
Solved A. Chem...
O CHEMICAL REACTIONS
Using molarity to find solute mass and solution volume
Caliyah v
A chemist adds 75.0 mL of a 0.0557 mM potassium permanganate (KMNO,) solution to a reaction flask. Calculate the mass in milligrams of potassium
permanganate the chemist has added to the flask. Be sure your answer has the correct number of significant digits.
mg
x10
olo
Ar
Explanatio
ck
20ZT MICoraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibility
nme
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning