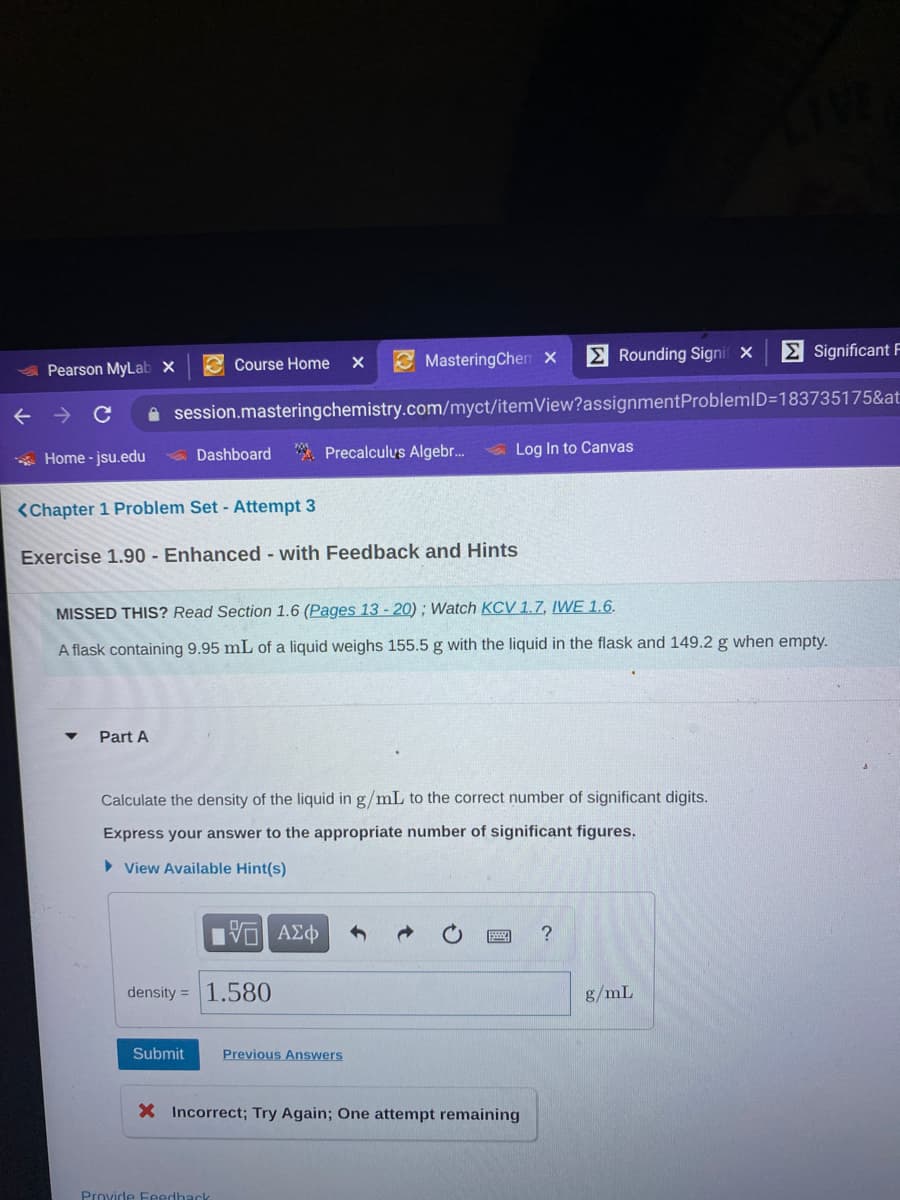
A flask 9.95 mL of a liquid 155.5 with the liquid in the flask 149.2 when em. Calculate the density of the liquid in g/mL to the correct number of significant digits.
A flask 9.95 mL of a liquid 155.5 with the liquid in the flask 149.2 when em. Calculate the density of the liquid in g/mL to the correct number of significant digits.
Chapter2: Basic Statistical Analysis With Excel
Section: Chapter Questions
Problem 12P
Related questions
Question
A flask 9.95 mL of a liquid 155.5 with the liquid in the flask 149.2 when em.
Calculate the density of the liquid in g/mL to the correct number of significant digits.
Transcribed Image Text:E Rounding Signif X
E Significant F
4Pearson MyLab x
e Course Home
MasteringChen x
session.masteringchemistry.com/myct/itemView?assignmentProblemID=183735175&at:
Dashboard Precalculus Algebr.
Log In to Canvas
* Home - jsu.edu
<Chapter 1 Problem Set - Attempt 3
Exercise 1.90 - Enhanced with Feedback and Hints
MISSED THIS? Read Section 1.6 (Pages 13 - 20); Watch KCV 1.7, IWE 1.6.
A flask containing 9.95 mL of a liquid weighs 155.5 g with the liquid in the flask and 149.2 g when empty.
Part A
Calculate the density of the liquid in g/mL to the correct number of significant digits.
Express your answer to the appropriate number of significant figures.
> View Available Hint(s)
density = 1.580
g/mL
Submit
Previous Answers
X Incorrect; Try Again; One attempt remaining
Provide Feedhack
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

