A mixture consisting of 15 mole % Phenol in water is to batch distilled at 260 torr, what fraction of the original batch is remains in the still when the total distillate contain 98mole % w water? What is the residues concentration? Change the feed compositions (5 values) and the distillate (5 values) , Draw the plot (xf vs xw) and (xf vs yD) , and write your illustrations. Equilibrium data: х% 1.54 4.95 6.87 7.73 19.63 28.44 H2O y% 41.1 79.7 82.7 84.4 89.91 91.05 Н20 2 9 5
A mixture consisting of 15 mole % Phenol in water is to batch distilled at 260 torr, what fraction of the original batch is remains in the still when the total distillate contain 98mole % w water? What is the residues concentration? Change the feed compositions (5 values) and the distillate (5 values) , Draw the plot (xf vs xw) and (xf vs yD) , and write your illustrations. Equilibrium data: х% 1.54 4.95 6.87 7.73 19.63 28.44 H2O y% 41.1 79.7 82.7 84.4 89.91 91.05 Н20 2 9 5
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter7: Extraction
Section: Chapter Questions
Problem 1Q
Related questions
Question
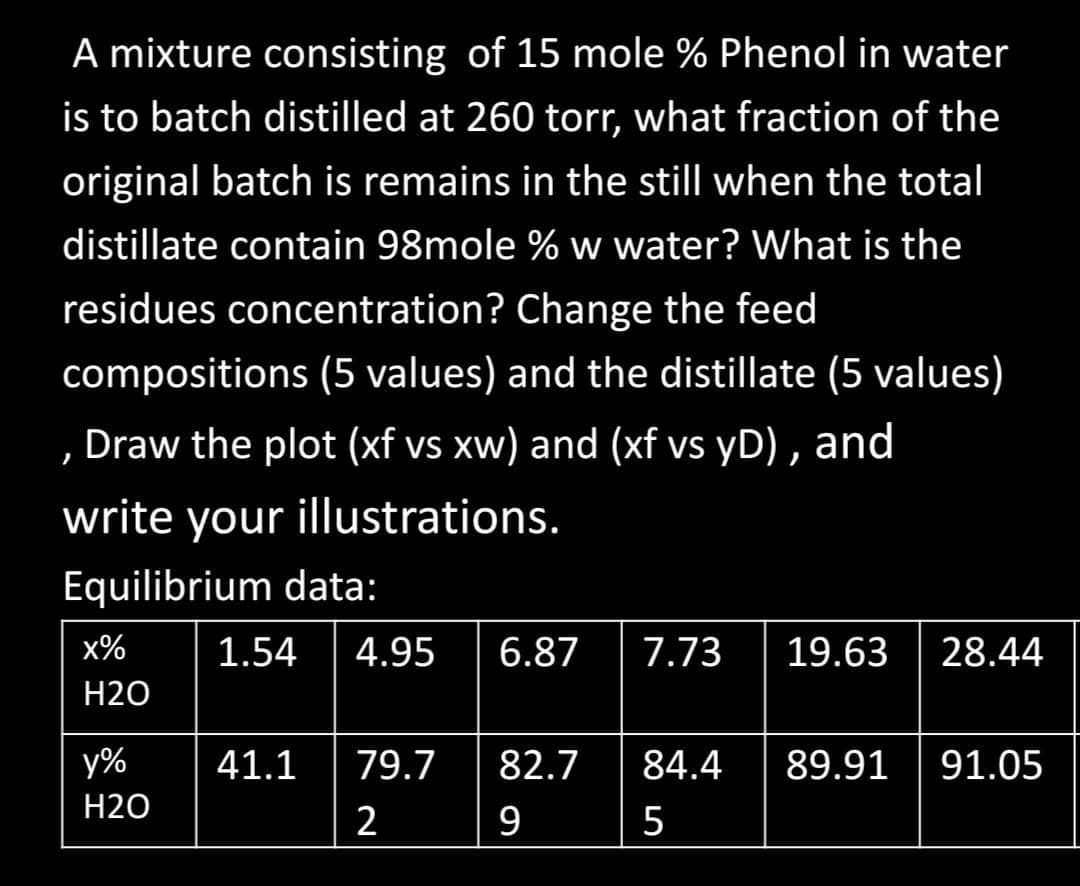
Transcribed Image Text:A mixture consisting of 15 mole % Phenol in water
is to batch distilled at 260 tor, what fraction of the
original batch is remains in the still when the total
distillate contain 98mole % w water? What is the
residues concentration? Change the feed
compositions (5 values) and the distillate (5 values)
, Draw the plot (xf vs xw) and (xf vs yD) , and
write your illustrations.
Equilibrium data:
x%
1.54
4.95
6.87
7.73
19.63
28.44
Н20
y%
41.1
79.7
82.7
84.4
89.91
91.05
Н20
2
9
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 8 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole



Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT