The solution may contain one or more of the following cations: Bit3 Mg+2 K+1 Ag+1 Pb+2 Nit2 Hg2+2 Reagen/t and Condition/s Observation/s A. To 20 drops of the unknown solution 3 drops 3M HCI was added; stirred and centrifuged. Added 1 more drop, stirred and centrifuged again white ppt colorless centifugate В white ppt. The precipitate was washed The centrifugate from A transferred into casserole, evaporated. The residue dissolved in 1 drop conc HCI, transferred to a test tube. Mixture in the test tube from C + 10 drops of 10% thioacetamide, 10 minutes in boiling water bath; centrifuged and decanted N/A no ppt very light tinge of yellow, clear liquid no ppt Precipitate from D + 5 drops 6M NaOH and 15 drops NazS, heated for 10 mins in water bath; centrifuged and decanted E colorless centifugate F Centrifugate from D + 4 drops NH4CI, alkalinized with 12M NH4OH no ppt 10% TA was added to the solution; heated for 10 mins in water bath; centrifuged and decanted no ppt ppt from E was dissolved in 5 drops conc HCl and made alkaline with 3M NaOH. Solid H2O2 no ppt was slowly added, with stirring; centrifuged and decanted G colorless liquid Centrifugate from F was evaporated until half volume, then + 3 drops 12M NH4OH and placed into a test tube. Mixture was heated in water bath for 3 minutes. About 15 drops of equal volumes of 3M (NH4)2CO3 and 95% ethanol solution were added to the heated mixture The final mixture in H was centrifuged and decanted N/A no ppt colorless centifugate purple color of flame no ppt formed in the hot mixture J The centrifugate from I was subjected to Flame Test K The washed ppt from B + 8 drops distilled water, then was heated for 3 minutes in a water bath with constant stirring, which was immediately centrifuged and decanted while hot, wherein 4 drops of K2Cr204 was added to the hot centrifugate The ppt from K + 10 drops NH4OH; stirred, centrifuged and decanted The centrifugate from L+ 3M HNO3 until acidic to blue litmus residue appears to be darkening colorless solution L
The solution may contain one or more of the following cations: Bit3 Mg+2 K+1 Ag+1 Pb+2 Nit2 Hg2+2 Reagen/t and Condition/s Observation/s A. To 20 drops of the unknown solution 3 drops 3M HCI was added; stirred and centrifuged. Added 1 more drop, stirred and centrifuged again white ppt colorless centifugate В white ppt. The precipitate was washed The centrifugate from A transferred into casserole, evaporated. The residue dissolved in 1 drop conc HCI, transferred to a test tube. Mixture in the test tube from C + 10 drops of 10% thioacetamide, 10 minutes in boiling water bath; centrifuged and decanted N/A no ppt very light tinge of yellow, clear liquid no ppt Precipitate from D + 5 drops 6M NaOH and 15 drops NazS, heated for 10 mins in water bath; centrifuged and decanted E colorless centifugate F Centrifugate from D + 4 drops NH4CI, alkalinized with 12M NH4OH no ppt 10% TA was added to the solution; heated for 10 mins in water bath; centrifuged and decanted no ppt ppt from E was dissolved in 5 drops conc HCl and made alkaline with 3M NaOH. Solid H2O2 no ppt was slowly added, with stirring; centrifuged and decanted G colorless liquid Centrifugate from F was evaporated until half volume, then + 3 drops 12M NH4OH and placed into a test tube. Mixture was heated in water bath for 3 minutes. About 15 drops of equal volumes of 3M (NH4)2CO3 and 95% ethanol solution were added to the heated mixture The final mixture in H was centrifuged and decanted N/A no ppt colorless centifugate purple color of flame no ppt formed in the hot mixture J The centrifugate from I was subjected to Flame Test K The washed ppt from B + 8 drops distilled water, then was heated for 3 minutes in a water bath with constant stirring, which was immediately centrifuged and decanted while hot, wherein 4 drops of K2Cr204 was added to the hot centrifugate The ppt from K + 10 drops NH4OH; stirred, centrifuged and decanted The centrifugate from L+ 3M HNO3 until acidic to blue litmus residue appears to be darkening colorless solution L
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.19QAP
Related questions
Question
In this unknown data sample, what is/are the cation present?
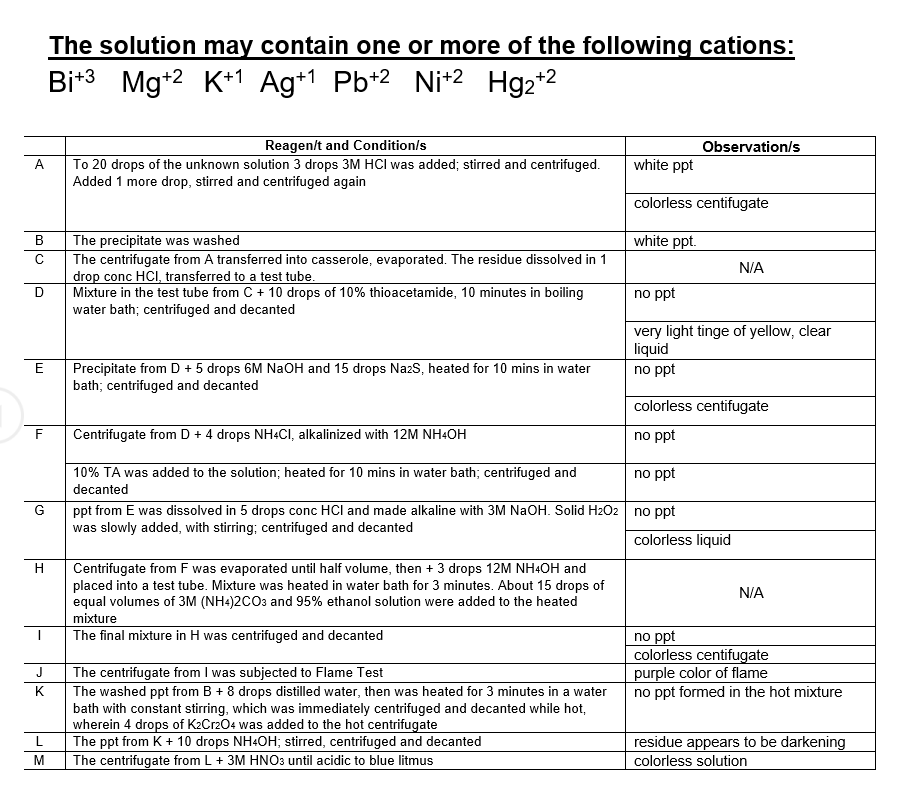
Transcribed Image Text:The solution may contain one or more of the following cations:
Bit3
Mg+2 K+1 Ag+1 Pb+2 Nit2
Hg2+2
Reagen/t and Condition/s
Observation/s
A.
To 20 drops of the unknown solution 3 drops 3M HCI was added; stirred and centrifuged.
Added 1 more drop, stirred and centrifuged again
white ppt
colorless centifugate
В
white ppt.
The precipitate was washed
The centrifugate from A transferred into casserole, evaporated. The residue dissolved in 1
drop conc HCI, transferred to a test tube.
Mixture in the test tube from C + 10 drops of 10% thioacetamide, 10 minutes in boiling
water bath; centrifuged and decanted
N/A
no ppt
very light tinge of yellow, clear
liquid
no ppt
Precipitate from D + 5 drops 6M NaOH and 15 drops NazS, heated for 10 mins in water
bath; centrifuged and decanted
E
colorless centifugate
F
Centrifugate from D + 4 drops NH4CI, alkalinized with 12M NH4OH
no ppt
10% TA was added to the solution; heated for 10 mins in water bath; centrifuged and
decanted
no ppt
ppt from E was dissolved in 5 drops conc HCl and made alkaline with 3M NaOH. Solid H2O2 no ppt
was slowly added, with stirring; centrifuged and decanted
G
colorless liquid
Centrifugate from F was evaporated until half volume, then + 3 drops 12M NH4OH and
placed into a test tube. Mixture was heated in water bath for 3 minutes. About 15 drops of
equal volumes of 3M (NH4)2CO3 and 95% ethanol solution were added to the heated
mixture
The final mixture in H was centrifuged and decanted
N/A
no ppt
colorless centifugate
purple color of flame
no ppt formed in the hot mixture
J
The centrifugate from I was subjected to Flame Test
K
The washed ppt from B + 8 drops distilled water, then was heated for 3 minutes in a water
bath with constant stirring, which was immediately centrifuged and decanted while hot,
wherein 4 drops of K2Cr204 was added to the hot centrifugate
The ppt from K + 10 drops NH4OH; stirred, centrifuged and decanted
The centrifugate from L+ 3M HNO3 until acidic to blue litmus
residue appears to be darkening
colorless solution
L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

