A particular reactant decomposes with a half-life of 121 s when its initial concentration is 0.354 M. The same reactant decomposes with a half-life of 239 s when its initial concentration is 0.179 M. Determine the reaction order. What is the value and units of the rate constant for this reaction? Units k = 0.17 Enter numeric value M-. - TOOLS x10
A particular reactant decomposes with a half-life of 121 s when its initial concentration is 0.354 M. The same reactant decomposes with a half-life of 239 s when its initial concentration is 0.179 M. Determine the reaction order. What is the value and units of the rate constant for this reaction? Units k = 0.17 Enter numeric value M-. - TOOLS x10
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.45PAE: The initial concentration of the reactant in a tirst-order reaction A —» products is 0.64 rnol/L and...
Related questions
Question
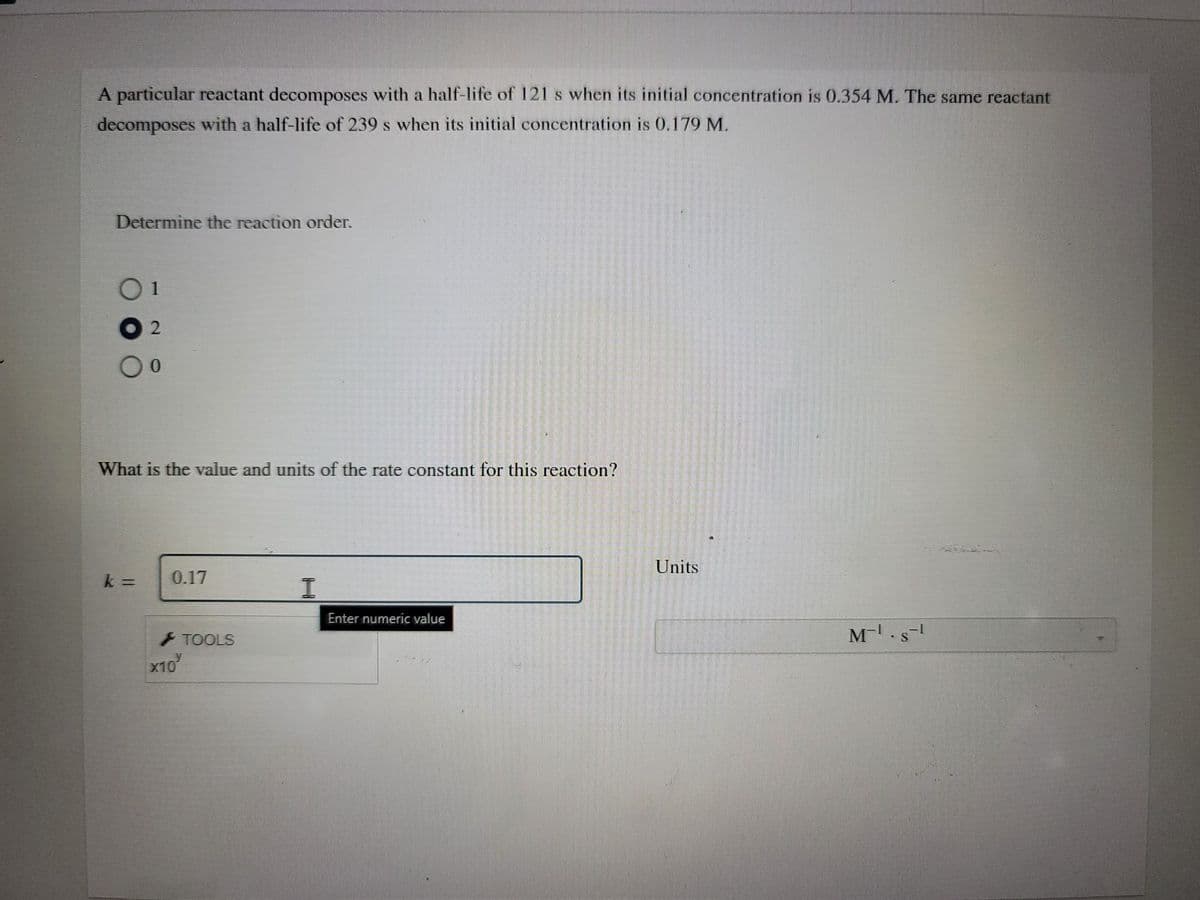
Transcribed Image Text:A particular reactant decomposes with a half-life of 121 s when its initial concentration is 0.354 M. The same reactant
decomposes with a half-life of 239 s when its initial concentration is 0.179 M.
Determine the reaction order.
01
What is the value and units of the rate constant for this reaction?
Units
0.17
Enter numeric value
TOOLS
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning