A particulate model for the chemical reaction resulting in the mixing of aqueous silver nitrate with aqueous potassium iodide is to be drawn, below. One (1) formula unit of each compound, and no more than six (6) water molecules in each starting solution and twelve (12) water molecules in the mixture, are used to show the dissolved states. AO legend to identify each ion. Water is drawn as a dipole o* 000 0. Show the bond-breaking of each substance and the bond-making that takes place to form the aqueous mixture and a precipitate, silver iodide. Panels A through E, below the scheme, show the different options for this scheme. Bond breaking Petaslum ledide solution Legend Band breaking Aqueou solution Agl Silver nitrate selution bond making (miture NO,. Panels: 4 Column A Column B The potassium iodide solution is best exemplified by The silver nitrate solution is best exemplified by 1. a. Panel D 2. b. Panel A 3. C. Panel E Separated potassium, iodide, and water particles (no interactions) best exemplified by Separated silver, nitrate, and water particles (no interactions) best exemplified by Mixture of potassium nitrate solution and silver iodide precipitate best exemplified by 4. d. Panel B 5. e. Panel C - D. 8.
A particulate model for the chemical reaction resulting in the mixing of aqueous silver nitrate with aqueous potassium iodide is to be drawn, below. One (1) formula unit of each compound, and no more than six (6) water molecules in each starting solution and twelve (12) water molecules in the mixture, are used to show the dissolved states. AO legend to identify each ion. Water is drawn as a dipole o* 000 0. Show the bond-breaking of each substance and the bond-making that takes place to form the aqueous mixture and a precipitate, silver iodide. Panels A through E, below the scheme, show the different options for this scheme. Bond breaking Petaslum ledide solution Legend Band breaking Aqueou solution Agl Silver nitrate selution bond making (miture NO,. Panels: 4 Column A Column B The potassium iodide solution is best exemplified by The silver nitrate solution is best exemplified by 1. a. Panel D 2. b. Panel A 3. C. Panel E Separated potassium, iodide, and water particles (no interactions) best exemplified by Separated silver, nitrate, and water particles (no interactions) best exemplified by Mixture of potassium nitrate solution and silver iodide precipitate best exemplified by 4. d. Panel B 5. e. Panel C - D. 8.
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 5P
Related questions
Question
please help me find the answers and fast, im being timed!!!
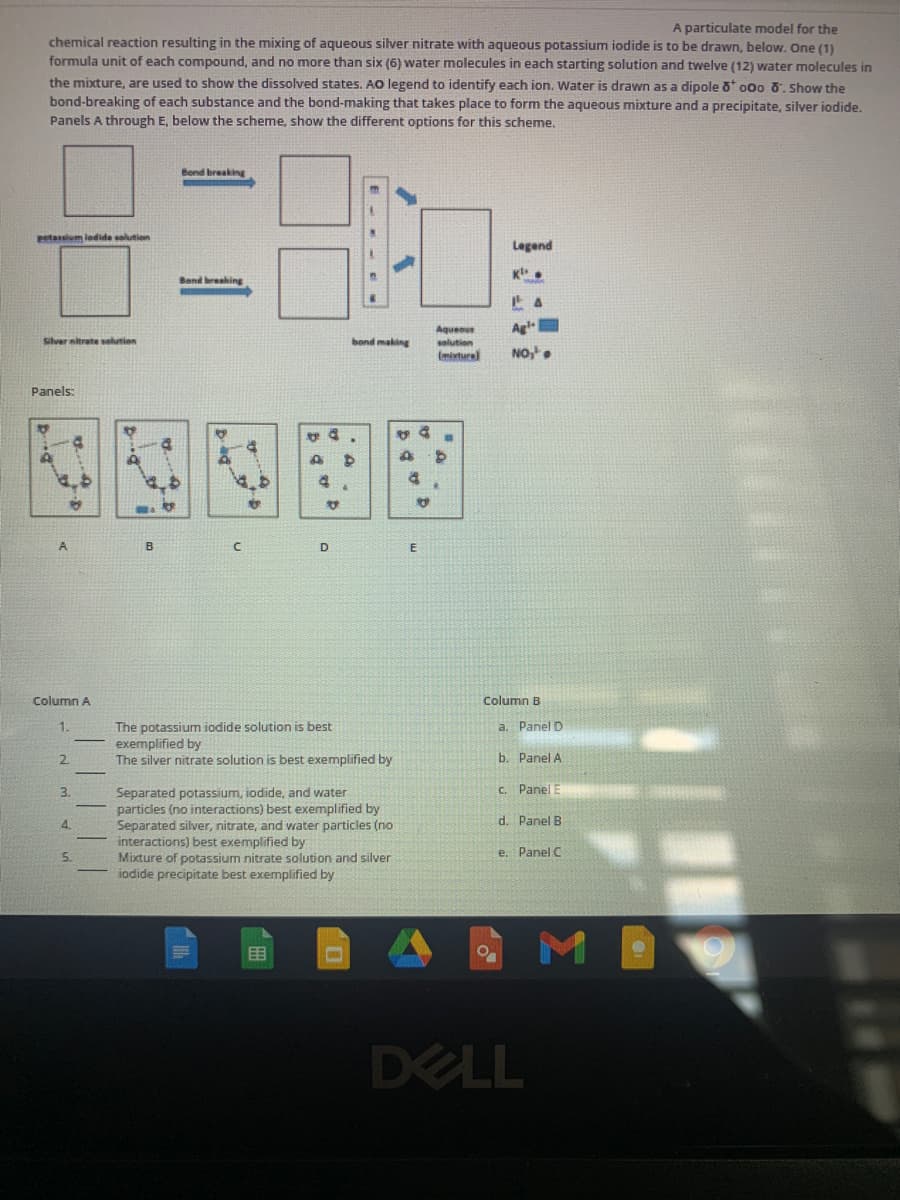
Transcribed Image Text:A particulate model for the
chemical reaction resulting in the mixing of aqueous siver nitrate with aqueous potassium iodide is to be drawn, below. One (1)
formula unit of each compound, and no more than six (6) water molecules in each starting solution and twelve (12) water molecules in
the mixture, are used to show the dissolved states. AO legend to identify each ion. Water is drawn as a dipole o 000 0. Show the
bond-breaking of each substance and the bond-making that takes place to form the aqueous mixture and a precipitate, silver iodide.
Panels A through E, below the scheme, show the different options for this scheme.
Bond breaking
Petaslum lodide solution
Legend
Band breaking
anoenby
Agl
Silver nitrate selution
bond making
solution
(miture
NO,
Panels:
A
B
D
Column A
Column B
The potassium iodide solution is best
exemplified by
The silver nitrate solution is best exemplified by
a. Panel D
2.
b. Panel A
3.
C. Panel
Separated potassium, iodide, and water
particles (no interactions) best exemplified by
Separated silver, nitrate, and water particles (no
interactions) best exemplified by
Mixture of potassium nitrate solution and silver
iodide precipitate
d. Panel B
4.
5.
e. Panel C
exemplified by
目
DELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax