A patient is suspected of having low stomach acid, a condition known as hypochloridia. To determine whether the patient has this condition, her doctors take a 20.00 mL sample of her gastric juices and titrate the sample with 1.73 x 10-4M KOH. The gastric juice sample required 4.82 mL of the KOH titrant to neutralize it. Calculate the pH of the gastric juice sample. Assume the sample contained no ingested food or drink which might otherwise interfere with the titration. 3.151 pH = %3D Incorrect For the patient to be suffering from hypochloridia, the pH of the gastric juices from the stomach must be greater than pH 4. Does the patient have hypochloridia? O yes O unable to determine
A patient is suspected of having low stomach acid, a condition known as hypochloridia. To determine whether the patient has this condition, her doctors take a 20.00 mL sample of her gastric juices and titrate the sample with 1.73 x 10-4M KOH. The gastric juice sample required 4.82 mL of the KOH titrant to neutralize it. Calculate the pH of the gastric juice sample. Assume the sample contained no ingested food or drink which might otherwise interfere with the titration. 3.151 pH = %3D Incorrect For the patient to be suffering from hypochloridia, the pH of the gastric juices from the stomach must be greater than pH 4. Does the patient have hypochloridia? O yes O unable to determine
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter14: Acids And Bases
Section: Chapter Questions
Problem 119QRT
Related questions
Question
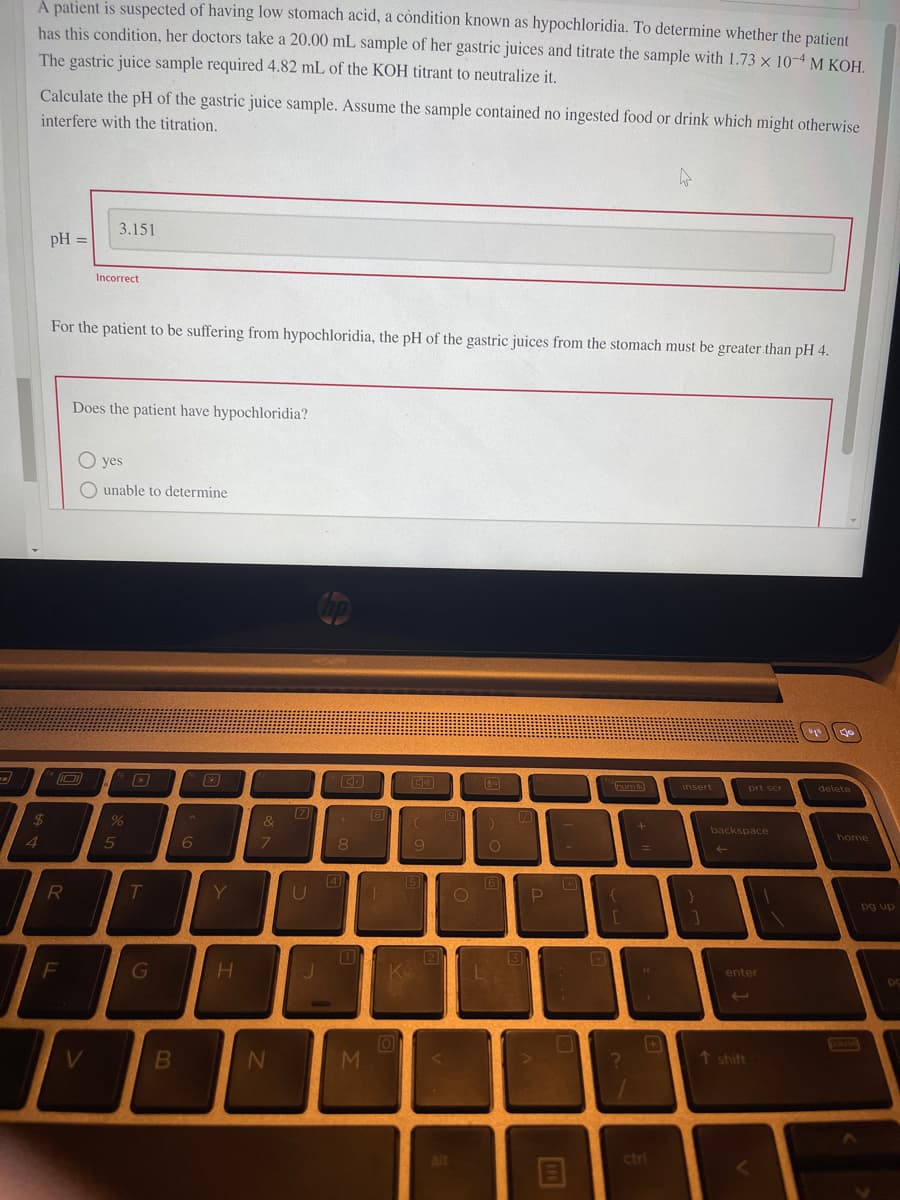
Transcribed Image Text:A patient is suspected of having low stomach acid, a cóndition known as hypochloridia. To determine whether the patient
has this condition, her doctors take a 20.00 mL sample of her gastric juices and titrate the sample with 1.73 x 10-4 M KOH.
The gastric juice sample required 4.82 mL of the KOH titrant to neutralize it.
Calculate the pH of the gastric juice sample. Assume the sample contained no ingested food or drink which might otherwise
interfere with the titration.
3.151
pH =
Incorrect
For the patient to be suffering from hypochloridia, the pH of the gastric juices from the stomach must be greater than pH 4.
Does the patient have hypochloridia?
O yes
O unable to determine
um
insert
prt scr
delete
&
backspace
home
4
4
RI
dn 6d
2
13
enter
F
G
↑ shift
ctri
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning