A solution is prepared by dissolving 20.5 g of NaOH in 130.0 g of water. The NaOH solution has a density of 1.15 g/mL. Part A What is the mass percent (m/m) of the NaOH solution? 13.6 % (m/m) Submit Previous Answers Correct Part B What is the total volume, in milliliters, of the solution? V = 131 mL Subimit Previous Answers Correct Part C What is the mass / volume percent (m/v) of the solution? HV ΑΣφ
A solution is prepared by dissolving 20.5 g of NaOH in 130.0 g of water. The NaOH solution has a density of 1.15 g/mL. Part A What is the mass percent (m/m) of the NaOH solution? 13.6 % (m/m) Submit Previous Answers Correct Part B What is the total volume, in milliliters, of the solution? V = 131 mL Subimit Previous Answers Correct Part C What is the mass / volume percent (m/v) of the solution? HV ΑΣφ
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.16QAP
Related questions
Question
100%
HELP! I’ve tried two different answers. I don’t know what I’m doing wrong!?
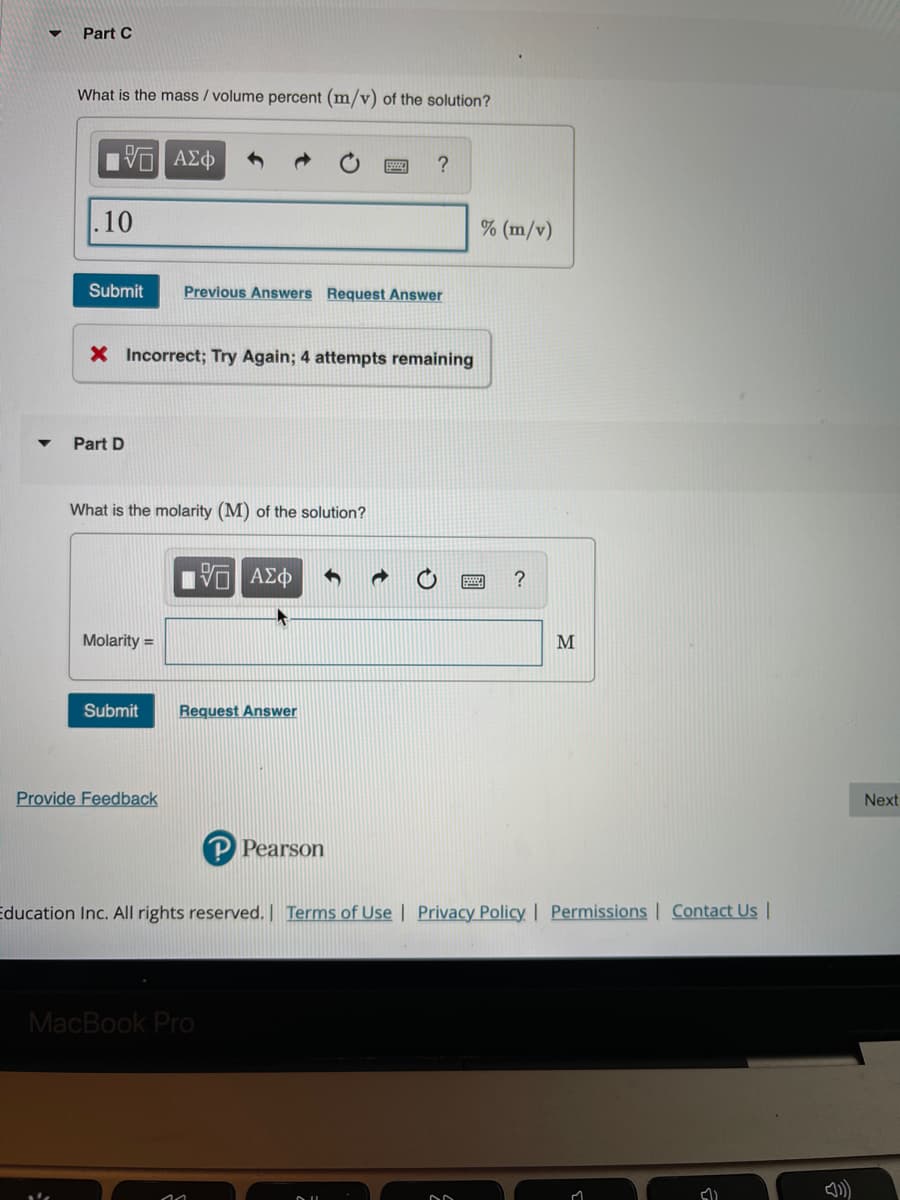
Transcribed Image Text:Part C
What is the mass / volume percent (m/v) of the solution?
.10
% (m/v)
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 4 attempts remaining
Part D
What is the molarity (M) of the solution?
?
Molarity =
M
Submit
Request Answer
Provide Feedback
Next
P Pearson
Education Inc. All rights reserved. Terms of Use | Privacy Policy | Permissions | Contact Us |
MacBook Pro
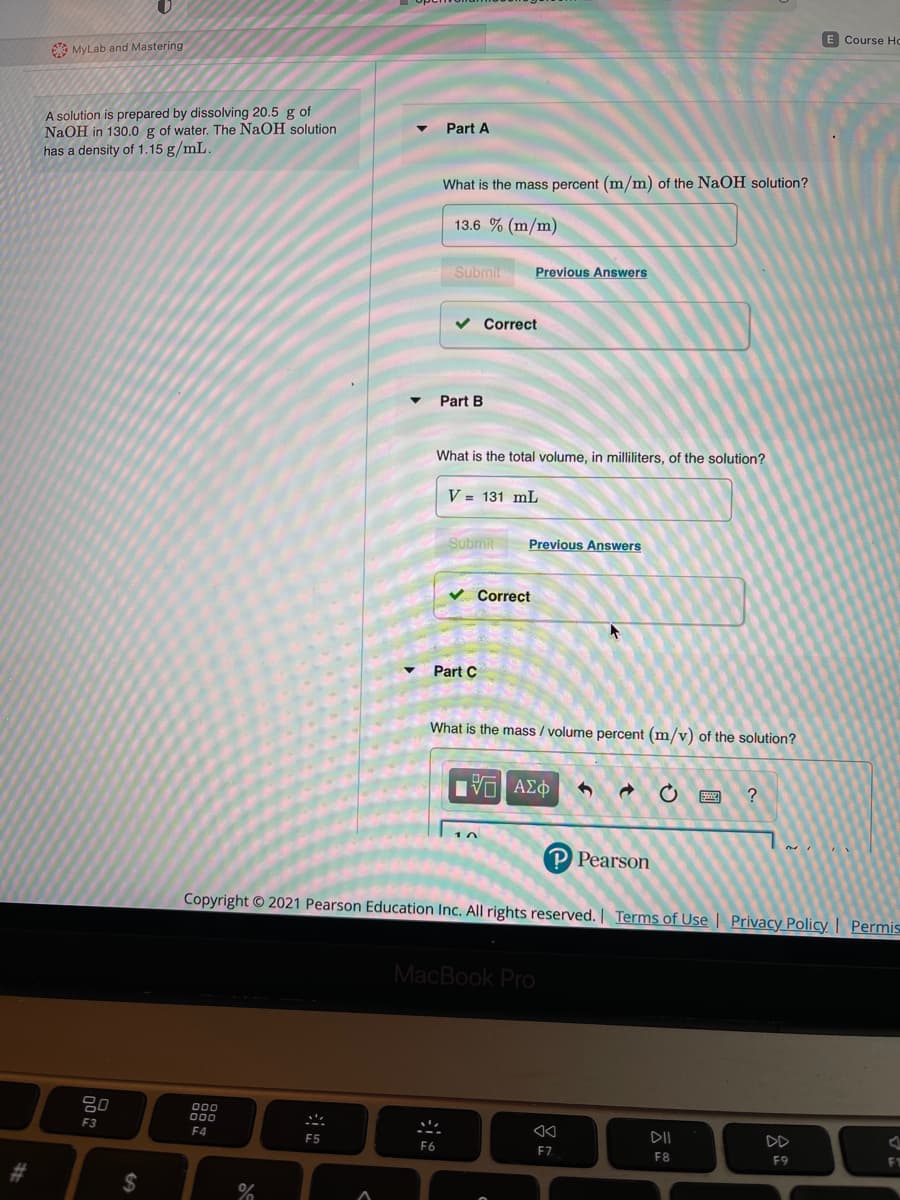
Transcribed Image Text:E Course Ho
MyLab and Mastering
A solution is prepared by dissolving 20.5 g of
NaOH in 130.0 g of water. The NaOH solution
has a density of 1.15 g/mL.
Part A
What is the mass percent (m/m) of the NaOH solution?
13.6 % (m/m)
Submit
Previous Answers
Correct
Part B
What is the total volume, in milliliters, of the solution?
V = 131 mL
Submit
Previous Answers
Correct
Part C
What is the mass / volume percent (m/v) of the solution?
P Pearson
Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permis
MacBook Pro
80
000
F3
F4
DII
DD
F5
F6
F7
F8
F9
%24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



