A solution is prepared by dissolving 8.28 g of ordinary sugar (sucrose, C12H2,011, molecular weight = 342 amu) in 34.7 g of water. Calculate the freezing point of the solution. Sucrose is a nonvolatile nonelectrolyte. Solvent T(°C) T:(°C) K (°C kg/mol) (°C kg/mol) Benzene, C,He 80.1 2.53 5.5 5.10 Camphor, C10H160 204 5.95 176 37.7 Cyclohexane, C,H12 80.88 2.79 6.50 20.2 Nitrobenzene, 210.8 5.24 5.7 8.1 C, H5NO2 Water, H20 100.0 0.512 0.00 1.86 -7.812 x °C
A solution is prepared by dissolving 8.28 g of ordinary sugar (sucrose, C12H2,011, molecular weight = 342 amu) in 34.7 g of water. Calculate the freezing point of the solution. Sucrose is a nonvolatile nonelectrolyte. Solvent T(°C) T:(°C) K (°C kg/mol) (°C kg/mol) Benzene, C,He 80.1 2.53 5.5 5.10 Camphor, C10H160 204 5.95 176 37.7 Cyclohexane, C,H12 80.88 2.79 6.50 20.2 Nitrobenzene, 210.8 5.24 5.7 8.1 C, H5NO2 Water, H20 100.0 0.512 0.00 1.86 -7.812 x °C
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter13: Solutions And Their Behavior
Section13.1: Units Of Concentration
Problem 1CYU: (a) If you dissolve 10.0 g (about one heaping teaspoonful) of sugar (sucrose, C12H22O11) in a cup of...
Related questions
Question
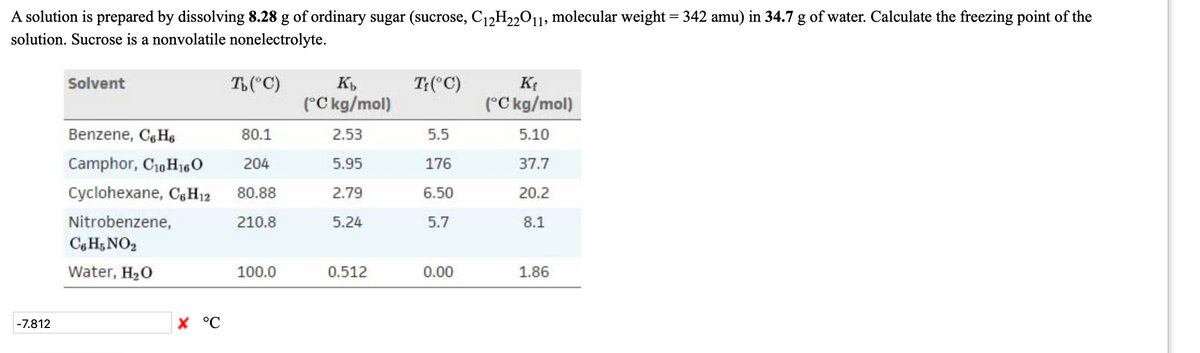
Transcribed Image Text:A solution is prepared by dissolving 8.28 g of ordinary sugar (sucrose, C12H22011, molecular weight = 342 amu) in 34.7 g of water. Calculate the freezing point of the
solution. Sucrose is a nonvolatile nonelectrolyte.
Solvent
T("C)
T:(°C)
(°C kg/mol)
(°C kg/mol)
Benzene, C, Hs
80.1
2.53
5.5
5.10
Camphor, C10 H160
204
5.95
176
37.7
Cyclohexane, CH12
80.88
2.79
6.50
20.2
Nitrobenzene,
210.8
5.24
5.7
8.1
Cg H5 NO2
Water, H20
100.0
0.512
0.00
1.86
-7.812
X °C
Expert Solution
Step 1
Depression in freezing point
by using this formula we can calculate freezing point

Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning