A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNsikr7j8P3jH-lijkPWvZoZLqKt1FLIq7wcPWKzBYGFE9IMFjRvsTdaQz2gLD2-UivJSKRyyqzt9fhmXWgWKvYgnx0... O MEASUREMENT Setting up the solution to a basic quantitative problem Ift The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant R. Suppose the osmotic pressure of a certain solution is measured to be 8.5 atm at an absolute temperature of 290. K. Write an equation that will let you calculate the molarity c of this solution. Your equation should contain only symbols. Be sure you define each symbol other than R. Your equation: Definitions of your symbols: = 8.5 atm D = 290. K Explanation Check O 2021 McGraw-Hill Education. All Riahts Reserved. Terms of Use I Privacy I Acc 6. éty
A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNsikr7j8P3jH-lijkPWvZoZLqKt1FLIq7wcPWKzBYGFE9IMFjRvsTdaQz2gLD2-UivJSKRyyqzt9fhmXWgWKvYgnx0... O MEASUREMENT Setting up the solution to a basic quantitative problem Ift The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant R. Suppose the osmotic pressure of a certain solution is measured to be 8.5 atm at an absolute temperature of 290. K. Write an equation that will let you calculate the molarity c of this solution. Your equation should contain only symbols. Be sure you define each symbol other than R. Your equation: Definitions of your symbols: = 8.5 atm D = 290. K Explanation Check O 2021 McGraw-Hill Education. All Riahts Reserved. Terms of Use I Privacy I Acc 6. éty
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
Transcribed Image Text:A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNsIkr7j8P3jH-lijkPWvZoZLqKt1FLlq7wcPWKzBYGFE9IMFjRvsTdaQz2gLD2-UivJSKRyyqzt9fhmXWgWKvYgnx0...
O MEASUREMENT
Setting up the solution to a basic quantitative problem
If
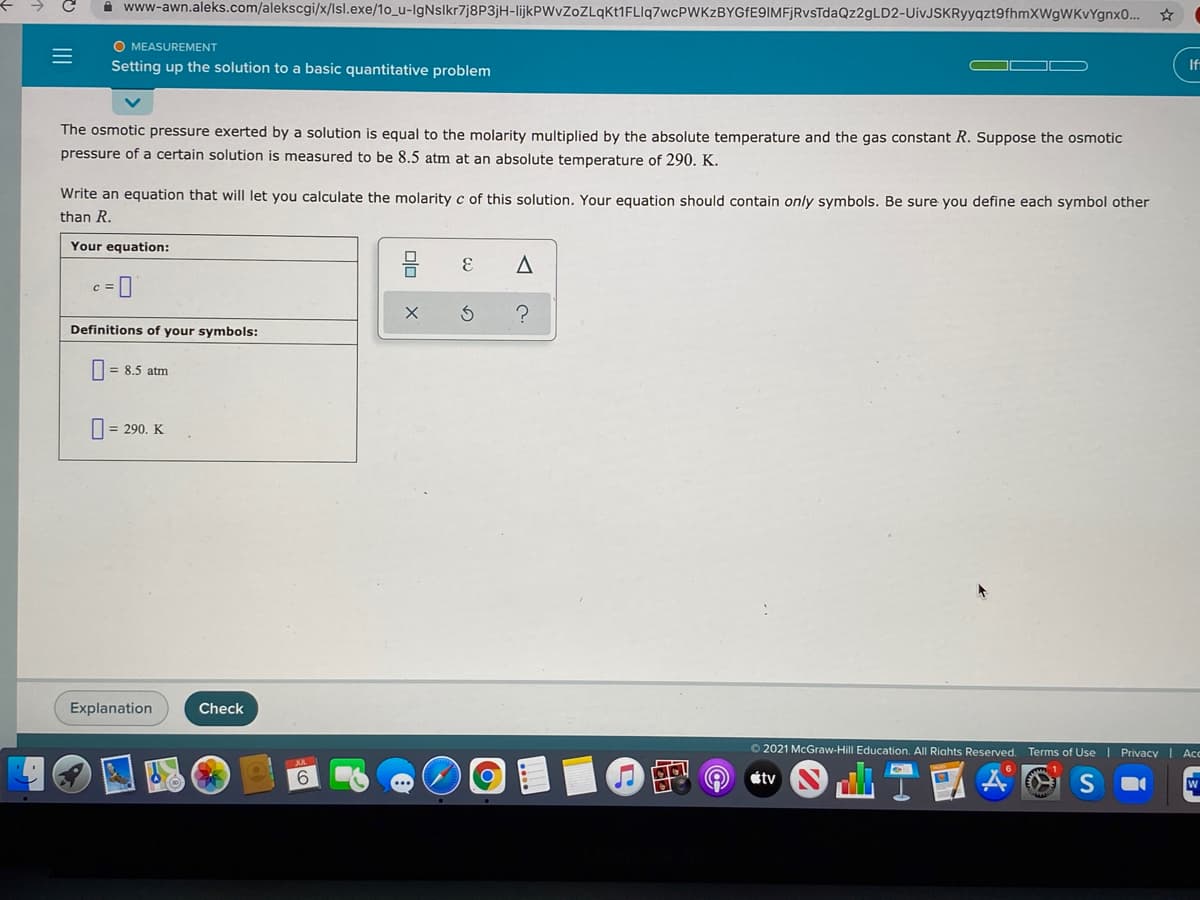
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant R. Suppose the osmotic
pressure of a certain solution is measured to be 8.5 atm at an absolute temperature of 290. K.
Write an equation that will let you calculate the molarity c of this solution. Your equation should contain only symbols. Be sure you define each symbol other
than R.
Your equation:
A
Definitions of your symbols:
= 8.5 atm
O = 290. K
Explanation
Check
O 2021 McGraw-Hill Education. All Riahts Reserved.
Terms of Use I Privacy I Acc
6.
étv
olo
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

