A. Dilution of Stock Solution Concentration of STOCK solution you prepared (M) 0.8 Volume of STOCK solution you prepared (mL) Volume of STOCK solution used for dilution (mL) Assigned concentration for the DILUTED solution (M) 0.16 Volume of DILUTED solution prepared (mL) Volume of water ADDED with the STOCK solution(mL)
A. Dilution of Stock Solution Concentration of STOCK solution you prepared (M) 0.8 Volume of STOCK solution you prepared (mL) Volume of STOCK solution used for dilution (mL) Assigned concentration for the DILUTED solution (M) 0.16 Volume of DILUTED solution prepared (mL) Volume of water ADDED with the STOCK solution(mL)
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter17: Principles Of Chemical Reactivity: Other Aspects Of Aqueous Equilibria
Section17.6: Equilibria Involving Complex Ions
Problem 1.1ACP
Related questions
Question
PLEASE FILL IN THE TABLES BELOW. THANK YOU!

Transcribed Image Text:Mass of active ingredient of the ascorbic acid tablet = 1000 mg
Amount of solute used = 1000 mg = 1.0 g
Assigned concentration of stock solution = 0.8 M
M
Wsolute (g)
*
MWsolute (g/mol)
V (L)
0.8 mol/L =
1.0 g
*
V
1
176.12 g/mol
1.0 g
0.8 mol/L * 176.12 g/mol
V =
= 7.1 * 10-3 L
= 7.1 mL
%3D
1000 mg of solute should be added to 7.1 mL water to get 0.8 M stock solution of ascorbic acid.
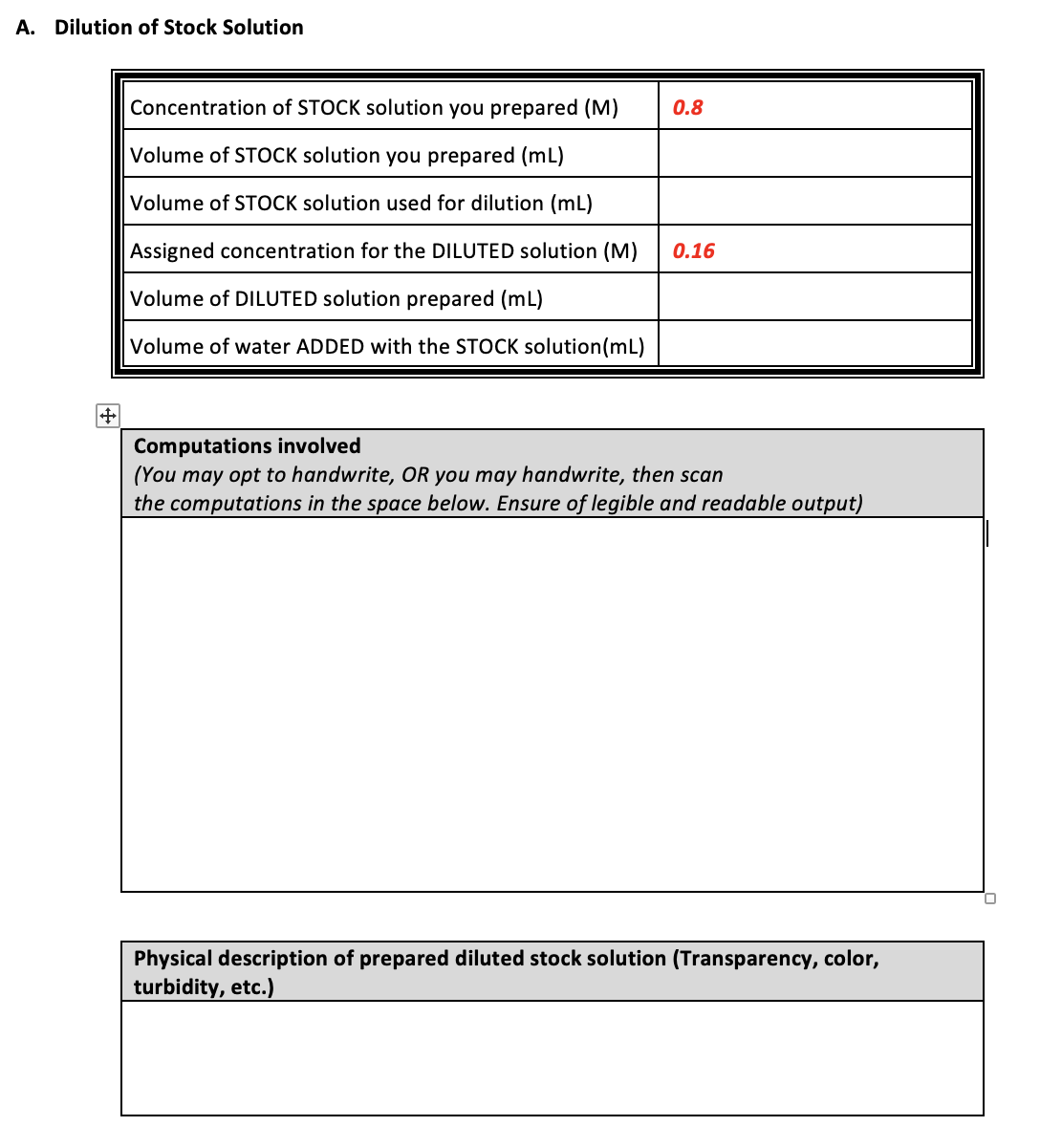
Transcribed Image Text:A. Dilution of Stock Solution
Concentration of STOCK solution you prepared (M)
0.8
Volume of STOCK solution you prepared (mL)
Volume of STOCK solution used for dilution (mL)
Assigned concentration for the DILUTED solution (M)
0.16
Volume of DILUTED solution prepared (mL)
Volume of water ADDED with the STOCK solution(mL)
Computations involved
(You may opt to handwrite, OR you may handwrite, then scan
the computations in the space below. Ensure of legible and readable output)
Physical description of prepared diluted stock solution (Transparency, color,
turbidity, etc.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
