а. Polymer plays important roles in our daily life and one of the examples is polypropylene. Referring to FIGURE Q3a, the monomer of polypropylene is Compound C, an alkene. This alkene can be produced from either Compound A or Compound B via dehydrohalogenation and dehydration reactions, respectively. Compound A Compound B Dehydrohalogenation Dehydration Route I Route II Compound C Radical Polymerization Polypropylene FIGURE Q3a i. Based on your knowledge in chemistry, elaborate TWO (2) applications of polypropylene and at least ONE (1) of its physical properties.
а. Polymer plays important roles in our daily life and one of the examples is polypropylene. Referring to FIGURE Q3a, the monomer of polypropylene is Compound C, an alkene. This alkene can be produced from either Compound A or Compound B via dehydrohalogenation and dehydration reactions, respectively. Compound A Compound B Dehydrohalogenation Dehydration Route I Route II Compound C Radical Polymerization Polypropylene FIGURE Q3a i. Based on your knowledge in chemistry, elaborate TWO (2) applications of polypropylene and at least ONE (1) of its physical properties.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter7: Bonding In Organic Molecules
Section: Chapter Questions
Problem 40AP: Consider the following proposed structures for benzene, each of which is consistent with the...
Related questions
Question
Please provide a detailed and clear explanation according to what question asked. Also, please provide a relevant diagram.
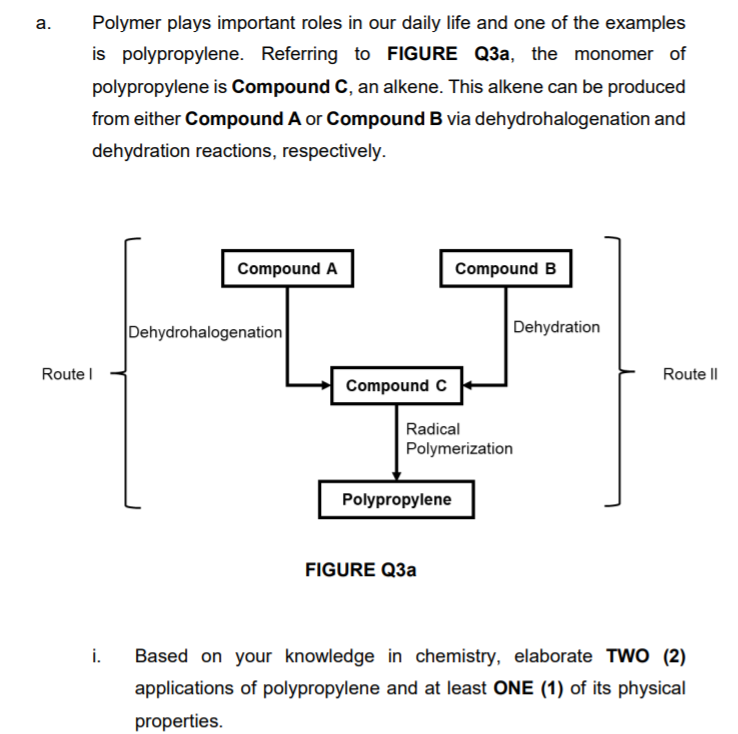
Transcribed Image Text:а.
Polymer plays important roles in our daily life and one of the examples
is polypropylene. Referring to FIGURE Q3a, the monomer of
polypropylene is Compound C, an alkene. This alkene can be produced
from either Compound A or Compound B via dehydrohalogenation and
dehydration reactions, respectively.
Compound A
Compound B
Dehydrohalogenation
Dehydration
Route l
Route I|
Compound C
Radical
Polymerization
Polypropylene
FIGURE Q3a
i.
Based on your knowledge in chemistry, elaborate TWO (2)
applications of polypropylene and at least ONE (1) of its physical
properties.

Transcribed Image Text:ii.
Select either Route I or Route II to synthesize polypropylene. Based
on the selected route, devise the synthesis of polypropylene from
its respective starting material by outlining the detailed reaction
mechanism.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning