A.) Quantum Numbers Fill the Table with the correct answer by replacing.the blue numbers.. Main Shell K M QUANTUM NUMBERS 1 2 3. 1. 2 3 -1,0,+1 Px, Py, Pz 2-1,0,+1,+2 (Types of d orbitala 2. m +/- %, +/- +/-% %, +/- % 10 12 12 Summary Notasion 1s 25 14 45 17 13 15 16 18 No. of Max Electrons 19 20 1. 1. -
A.) Quantum Numbers Fill the Table with the correct answer by replacing.the blue numbers.. Main Shell K M QUANTUM NUMBERS 1 2 3. 1. 2 3 -1,0,+1 Px, Py, Pz 2-1,0,+1,+2 (Types of d orbitala 2. m +/- %, +/- +/-% %, +/- % 10 12 12 Summary Notasion 1s 25 14 45 17 13 15 16 18 No. of Max Electrons 19 20 1. 1. -
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter5: Quantum Mechanics And Atomic Structure
Section: Chapter Questions
Problem 26P: Suppose that the spin quantum number had three allowed values (ms=0,+12,12) . Give the atomic...
Related questions
Question
Please help me answer this
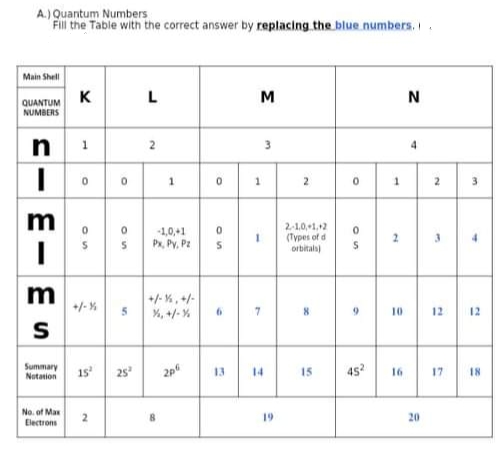
Transcribed Image Text:A.) Quantum Numbers
Fill the Table with the correct answer by replacing the blue numbers..
Main Shell
K
M
QUANTUM
NUMBERS
1
2
3.
1.
1.
2
3
-1,0,+1
Px, Py, Pz
2-1,0,+1,12
(Types of d
orbitals)
2.
m
+/-%
+/- %, +/-
%, +/- %
10
12
12
Summary
Notation
15
25
13
14
45?
17
18
15
16
No. of Max
19
20
Electrons
2.
1.
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning