a. Write balance molecular equations for the formation of copper(Il) nitrate, copper(II) carbonate, and copper(Il) sulfate as seen in this lab. (Begin with 3 moles of Cu (s) in the first equation.)
a. Write balance molecular equations for the formation of copper(Il) nitrate, copper(II) carbonate, and copper(Il) sulfate as seen in this lab. (Begin with 3 moles of Cu (s) in the first equation.)
Chapter17: Spontaneity, Entropy, And Free Energy
Section: Chapter Questions
Problem 99CWP: The following reaction occurs in pure water: H2O(l)+H2O(l)H3O+(aq)+OH-(aq) which is often...
Related questions
Question
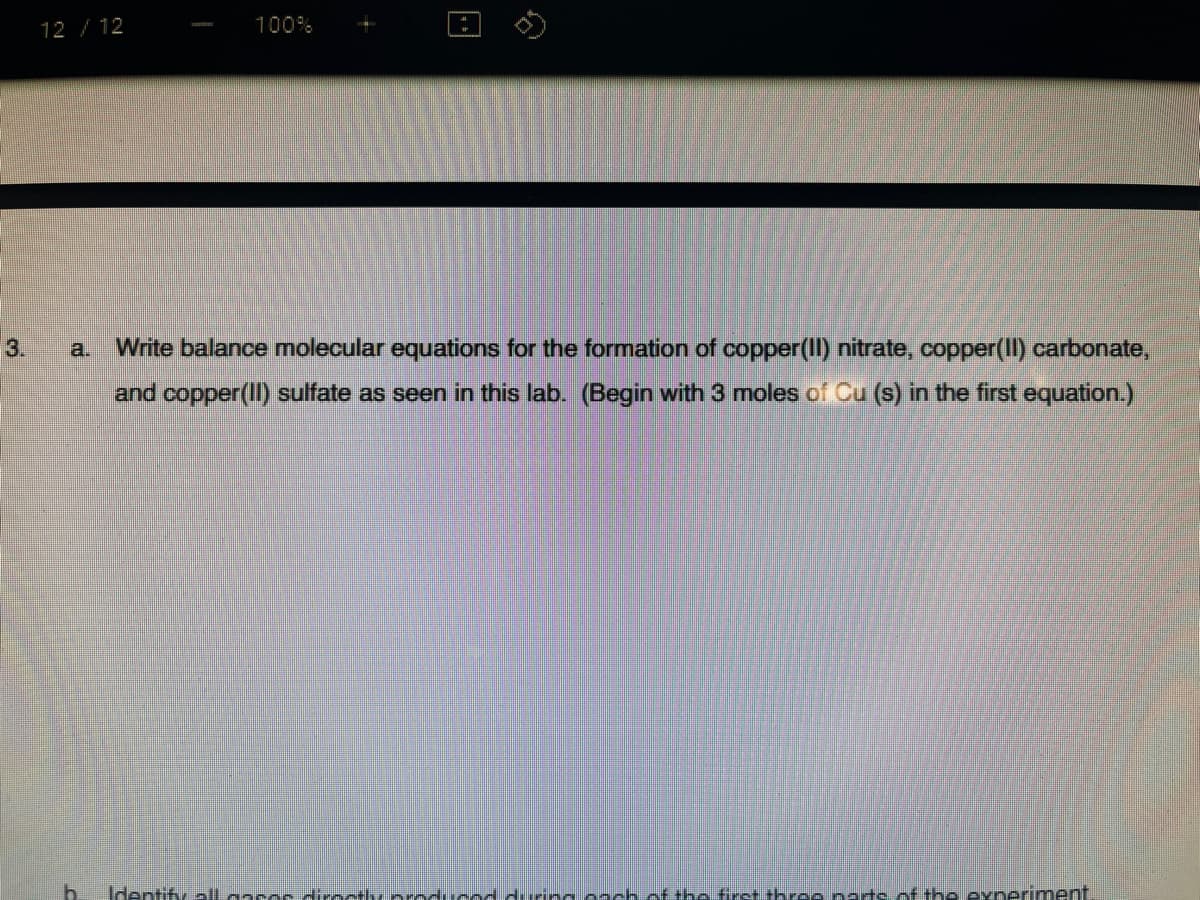
Transcribed Image Text:12 /12
100%
3.
a.
Write balance molecular equations for the formation of copper(II) nitrate, copper(II) carbonate,
and copper(II) sulfate as seen in this lab. (Begin with 3 moles of Cu (s) in the first equation.)
b.
Identify llaasesdirocthy roducadduring o-ch of the first th ce natsof the exneriment
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning